��Ŀ����
����Ŀ��������ͼ�������ش��������⣮
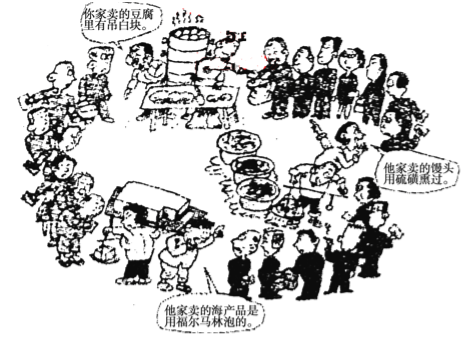
��1��������ӳ������ȵ������� ��
��2������ǣ�S��Ѭ����ͷ�����SO2��д���йط�Ӧ�Ļ�ѧ����ʽ�� ��SO2��ˮ���� ���ѧʽ���������ʶ�����ϵͳ�к���
��3���ø������֣���ȩˮ��Һ�����ݺ���Ʒ���ӳ��䱣���ڣ�ԭ���� ���������ļ�ȩ�������ǻ��θ�����Ȳ��䣻
��4�����飨CH3O3SNa���㷺Ӧ����ӡȾ��ҵ������������ʳƷ���Ӽ�����������ʱ�������·�Ӧ��6CH3O3SNa+3H2O![]() 4NaHSO3+2HCOONa+2H2S��+HCOOH+3X����X�Ļ�ѧʽΪ ��
4NaHSO3+2HCOONa+2H2S��+HCOOH+3X����X�Ļ�ѧʽΪ ��
���𰸡�
��1��ʳƷ��ȫ���⣻
��2��S+O2![]() SO2��H2SO3��
SO2��H2SO3��
��3����ȩʹϸ���������ʱ��ԣ�
��4��CH4O
��������
���������
��1�������к��е��顢��ͷ�����Ѭ��������Ʒ�ø������ֽ��ݶ�����ʳƷ�йصİ�ȫ���⣻
��2�����ڿ�����ȼ�����ɶ���������������ˮ���������
��3����ȩ��Һ��ʹ������ʧȥԭ�е��������ܶ��ӳ�ʳƷ�ı����ڣ�
��4�����������غ㶨�ɿ�֪��ѧ��Ӧǰ��Ԫ�ص����ࡢԭ�ӵ�������������䣬�����Աȷ�Ӧǰ���Ԫ�ص�ԭ�Ӹ����ɵ�X�Ļ�ѧʽΪCH4O���ʴ�Ϊ����1��ʳƷ��ȫ���⣻��2��S+O2![]() SO2��H2SO3����3����ȩʹϸ���������ʱ��ԣ���4��CH4O
SO2��H2SO3����3����ȩʹϸ���������ʱ��ԣ���4��CH4O

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�����Ŀ����ȥ���������е��������ʣ���ѡ�õ��Լ��Ͳ�����������ȷ����
ѡ�� | ���� | ���� | �Լ� | �������� |
A | MnO2 | KCl | ����ˮ | �ܽ⡢���ˡ��������ᾧ |
B | CO2 | H2 | �������� | ��ȼ |
C | NaOH��Һ | Ca��OH��2 | ����Na2CO3��Һ | ���� |
D | ϡ���� | ���� | ������������Һ | ���� |