��Ŀ����
����Ŀ���о�������ȼ��ʵ����������ʶˮ����ɵĿ�ʼ��ʵ�����г���п����ϡ���ᷴӦ���Ʊ��������������ϣ�Ũ���������ˮ��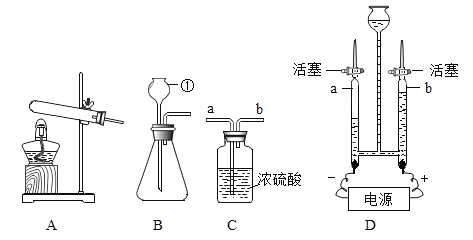
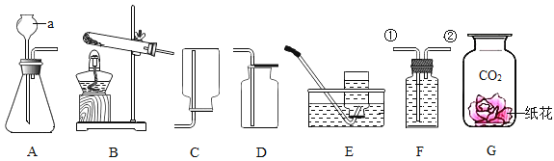
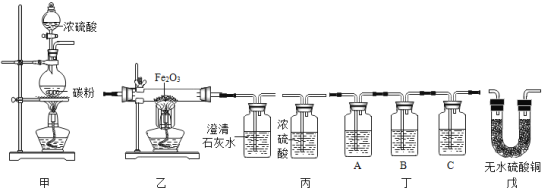
��1�������ٵ�������_____��ʵ������ȡ�����ķ���װ����_____������ĸ����Ҫ�ռ������������������Ҫ��Cװ����_____ ���a����b�����ܿڽ��롣
��2��ij��ѧ��ȤС���ͬѧ�á���ⷨ��֤����ˮ����ɣ���ͼD��ʾ����װ���з�����Ӧ�Ļ���������_____������b����ȼ�ŵ�ľ���ڲ����ܼ���ڼ��鷴Ӧ���������壬������������_____��
��3��ͬѧ�Dz������ϵ�֪����������һʵ��֤����ˮ����ɡ�����ˮ����ͨ��һ�������պ������ǹ�ܣ�����õ���������ͬʱǹ�ܱ����к�ɫ���壨���������������ɡ��÷�Ӧ�Ļ�ѧ����ʽΪ_____��
������̽��������ȤС���ͬѧ������������������̽��������ȡ7֧�Թܣ�����ʢˮ90%�������������80������������ˮ���ռ�����������ֱ���Թܿ��ƽ��ƾ��ƻ��棬ʵ���������ʾ��
��� | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
�������������%�� | 90 | 80 | 70 | 50 | 20 | 10 | 5 |
�������������%�� | 10 | 20 | 30 | 50 | 80 | 90 | 95 |
��ȼ���� | ����ȼ�� | ����ȼ�� | ���ı����� | ǿ�ı����� | ǿ�ı����� | ���ı����� | ��ȼ�ղ����� |
����������Ϣ������������⣺
��4���ڻ�����屬ը��Χ�ڣ����������������ΧԼΪ_____��
��5�����ȼ�ջ�ȼ������������ʶ��_____��
���𰸡�����©�� B a �ֽⷴӦ ȼ�ŵ�ľ��ȼ�յĸ��� 3Fe+4H2O Fe3O4+4H2 10%-70% ��Ӧ����������Ũ��Ҫ��һ����Χ�����ʲ��ܳ��ȼ�գ�������Ũ��Խ�ߣ���Ӧ��һ��Խ���ң��������ɣ�
Fe3O4+4H2 10%-70% ��Ӧ����������Ũ��Ҫ��һ����Χ�����ʲ��ܳ��ȼ�գ�������Ũ��Խ�ߣ���Ӧ��һ��Խ���ң��������ɣ�
��������
��1�������ٵ������dz���©����ʵ������ȡ��������п��ϡ���ᷴӦ��ȡ���÷�Ӧ�ķ�Ӧ���ǹ�����Һ�壬����Ҫ���ȣ�ѡ�õķ���װ����B��Cװ���е�Ũ���������ˮ�ԣ��Ҳ���������Ӧ������������������Ϊ��ʹ������Ũ�����ֽӴ���������Ҫ��Cװ����a�ܿڽ��룻
��2��ij��ѧ��ȤС���ͬѧ�á���ⷨ��֤����ˮ����ɣ�ˮͨ��ʱ�ֽ��������������������Ƿ�Ӧ����һ�������������ֵķ�Ӧ����ͼD��ʾ����װ���з�����Ӧ�Ļ��������ǷֽⷴӦ��ˮͨ��ʱ�����������������������������������Ϊ1��2������b���Դ����������������������������������������ȼ�ԣ�����b����ȼ�ŵ�ľ���ڲ����ܼ���ڼ��鷴Ӧ���������壬������������ȼ�ŵ�ľ��ȼ�յĸ�����
��3��ͬѧ�Dz������ϵ�֪����������һʵ��֤����ˮ����ɡ�����ˮ����ͨ��һ�������պ������ǹ�ܣ�����õ���������ͬʱǹ�ܱ����к�ɫ���壨���������������ɡ��÷�Ӧ�Ļ�ѧ����ʽΪ3Fe+4H2O Fe3O4+4H2��
Fe3O4+4H2��
��4�����ݱ����е����ݿ�֪���ڻ�����屬ը��Χ�ڣ����������������ΧԼΪ10%-70%��
��5����������ʵ������ȼ�ջ�ȼ������������ʶ�Ƿ�Ӧ����������Ũ��Ҫ��һ����Χ�����ʲ��ܳ��ȼ�գ�������Ũ��Խ�ߣ���Ӧ��һ��Խ���ң���

����Ŀ�������ڻ�ѧ������������ճ������У����ǹ㷺���ڶ��ַdz���Ҫ�����ʡ�����Գ���������������IJ������ʽ���̽����
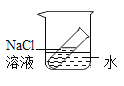
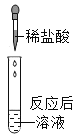
�ٽ�Ũ���������ɫʯ����ֽ�ϣ��۲쵽_______����ʱ��˵��Ũ�������______�����ʡ�
����ͼ��ʾ����ˮ����װ��Ũ�������ƿ��(��ƿ��ľ�����ۻ���ʯ��ճ��һ��)���۲쵽Һ���Ľ����ݴ˷��������й���Ũ�����������ȷ����____��
Aϡ��Ũ����ʱ��һ��Ҫ��Ũ���Ỻ��ע��ˮ��
Bϡ��Ũ����ʱ��һ��Ҫ��ˮ����ע��Ũ������
CŨ����������ˮ���Խ���ƿ��ľ������������
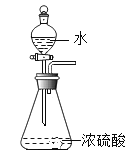
����ǽ�����Ӧ�������Ũ������뵽װ��̼�۵�Բ����ƿ�У������������ȸû����д������ݲ������ֶԵ���������ɷֽ�����֤��
���������ϣ�
��̼��Ũ�����ڼ���ʱ����������ˮ֮�⣬�����ɶ��������̼��һ�������
�ڶ�������Ͷ�����̼����ʹ����ʯ��ˮ����ǣ����У�����������ʹ�Ϻ�ɫ�����Ը��������Һ��ɫ��
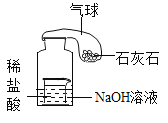
��ʵ����֤��ij��ѧ��ȤС������ʦָ�����������ͼʵ��װ�ý�����֤��
��ܰ��ʾ����װ�õ�A��B��C�о�װ���Ϻ�ɫ�����Ը��������Һ
��Ϊ����ɶԵ����������֤���뽫����װ�ý�����ȷ�����ӣ�����___������____���� (�����)��
�����������е�������ݣ�
ʵ������ | ʵ����� |
���й۲쵽������___________ | ֤��̼��Ũ������ȷ�Ӧ����̼���������Ƕ�����̼������һ����̼ |
��װ��A����Һ��ɫ����װ��C����Һ����ɫ | ֤����������___________��װ��C��������___________�� |
���а�ɫ������� | ֤����������ˮ���� |
����ý��ۣ�д��̼��Ũ������ȷ�Ӧ�Ļ�ѧ����ʽ��_______________��
��֪ʶ��չ����Ӧֹͣ��ͬѧ�ǶԷ�Ӧ���ʣ����ɷݺܺ��棬�����������ʵ�飺
ʵ�鲽�� | ʵ������ | ʵ����� |
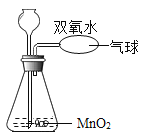
���ˣ�ȡ����ϴ�Ӹ����ú�ɫ���壬����ȼ�ճ��м��ȣ�Ѹ����������ƿ�У�����Ӧ����������������ʯ��ˮ���� | ����ʯ��ˮ���� | ��ɫ����Ϊ̼�� |
ȡ��Һ���Թ��У��μ���ɫʯ����Һ����μ�������������Һ���ߵα��� | ��ɫʯ����Һ��죬�а�ɫ������������Һ�ֱ���ɫ | ��Һ�ɷ������� |
��ʵ����ۣ�С����Ϊ��ʣ�������Ҫ�ɷ���̼�ۺ�ϡ���ᡣС����Ϊ����Ӧ�����ܷ�Ӧ������ͬʱ���ڣ����ԣ�С����뷨�Ǵ���ġ�����Ϊ____________���뷨����ȷ�ġ�������Ϊ_______________��
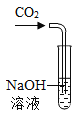
����Ŀ��.ij��ѧ��ȤС���ͬѧ�о�CO2ͨ��NaOH��Һ�Ƿ�����Ӧ���������������ʵ�顣����������ǵ�̽��������ش��й����⡣
�������ʵ�飩
ʵ����� | ����һ | ����� |
ʵ��� |
|
|
ʵ��� |
|
|
��̽������ۣ�
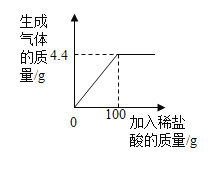
��1��ʵ��I�IJ�����в�����ʵ��������_________����Ӧ�Ļ�ѧ����ʽΪ_______________��
��2��ʵ���IJ�����в�����ʵ��������_____________��
��3����ʵ��I��ʵ���IJ���һ�У�CO2��NaOH��Һһ�������˻�ѧ��Ӧ����ȴ���������Ե�����ԭ����______________��
����չ���죩��֪20��ʱNaOH��Na2CO3��ˮ�к;ƾ��е��ܽ�����£�
��ˮ�е��ܽ��/g | �ھƾ��е��ܽ��/g | |
NaOH | 109 | 17.3 |
Na2CO3 | 21.8 | <0.01 |
������������Ϣ���ʵ���֤��CO2ȷʵ��NaOH�����˻�ѧ��Ӧ��
��1���û�ѧ����ʽ��ʾʵ����ԭ����____________________��
��2��ʵ������Ҫ������_____________________��
��������˼��ͨ��ʵ��I~���֪����֤����������ķ�Ӧ�Ƿ��������Բ��ü���������ķ������������о�һ�ּ��鷽����______��