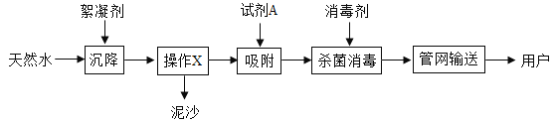
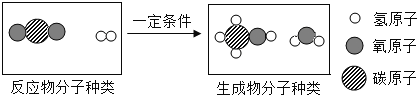
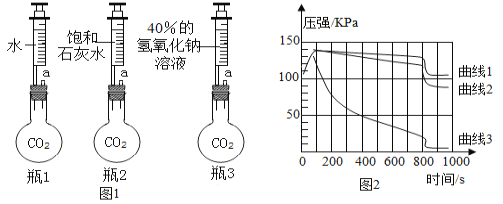
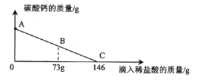
��Ŀ����
����Ŀ�������д����л�ѧ���ش��������⣺
��1��ͭ�������ߣ�����Ϊͭ����_______________�ԡ�
��2����ϴ�ྫϴȥ�;��ϵ����ۣ���ԭ����______________��
��3����Ȼ���ڿ�����ȼ�գ�������������_________________��
��4�������һ�ִ���ϲ����ʳ�ģ����и��������ʡ�ά���ء���ά�ء����������ǣ����⺬�зḻ������п���Ƶ�Ԫ�أ���������ȱ����______________��ѡ����ĸ����
a ƶѪ֢ b ���Ͳ� c ٪��֢ d ��״�ټ���
��5����������ˮʱ�����ö������������������仯ѧʽΪ_______________��
��6��һ����̼��̼Ԫ�صĻ��ϼ�Ϊ+2��_________��
���𰸡������� �黯���� ��ȼ�� a ClO2 ![]()
��������
��1��ͭ�������ߣ�����Ϊͭ���е����ԡ�
��2����ϴ�ྫϴȥ�;��ϵ����ۣ���ԭ����ϴ�ྫ���黯���ã�����ʹ���۷�ɢ��ˮ�С�
��3����Ȼ���ڿ�����ȼ�գ���������������ȼ�ԡ�
��4����������ȱ����ƶѪ֢����ѡa��
��5����������ˮʱ�����ö������������������仯ѧʽΪClO2��
��6��Ԫ�ػ��ϼ۵ı�ʾ������ȷ��������������Ҫ�����Ԫ�صĻ��ϼۣ�Ȼ�����仯ѧʽ��Ԫ�ص��Ϸ��������ź����ֱ�ʾ����������ǰ�������ں�һ����̼��̼Ԫ�صĻ��ϼ�Ϊ+2��![]() ��
��