��Ŀ����
�������۹ܵ��г���ۼ��ж���������������¹ʣ�ijѧϰС����֪���������۹ܵ����ijɷ���ʲô������ͨ���������ϵ�֪��
I���������۹ܵ��п��ܺ��нϴ�����CO��CO2��H2S��CH4�����壮
��H2S�����о綾������CuSO4��Һ��Ӧ���ɺ�ɫ������
��CO2�ᱻNaOH��Һ���գ� CH4��CO������CuSO4��Һ��ʯ��ˮ�Լ�NaOH��Һ��Ӧ��
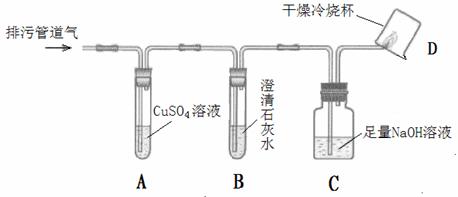
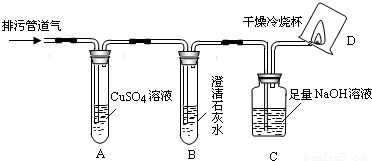
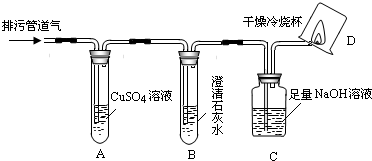
��С���������ͼ��ʾ��װ�ò�����̽�����г�������ʡ�ԣ���

��ش��������⣺
��1��װ��A�г��ֺ�ɫ���ǣ�˵�����۹ܵ����к���______���ѧʽ����
��2��װ��B�г��ְ�ɫ���ǣ�˵�����۹ܵ����к���______���ѧʽ�����˷�Ӧ�Ļ�ѧ����ʽΪ______��
��3��װ��D�пɼ����棬������ձ��ڱ���ˮ�����֣�˵�����۹ܵ����к���______���ѧʽ�����˷�Ӧ�Ļ�ѧ����ʽΪ______����Ҫ��֤������ȼ�պ����һ�ֲ���ɽ��еIJ����ǣ�Ѹ�ٰ��ձ������������ձ���ע��______����
��4����ͬѧ��������ȼ�ղ�����ˮ�Ͷ�����̼�����������㣻�� ��______����ԭ��Ʒ���к���CO������ԭ��Ʒ���оͲ���CO�����밴��˼·���������������գ���
��______����ԭ��Ʒ���к���CO������ԭ��Ʒ���оͲ���CO�����밴��˼·���������������գ���
�⣺
��1��װ��A��������ͭ��Һ���������ⷴӦ���ɺ�ɫ�����������ֺ�ɫ����֤�����۹ܵ��������⣬��ѧʽΪH2S��
��2��װ��B���dz����ʯ��ˮ��������̼��ʹ������ǣ���Bװ�ó��ְ�ɫ���ǣ�˵�����۹ܵ�����������̼����Ӧ��ѧ����ʽΪCO2+Ca��OH��2�TCaCO3��+H2O��
��3�����ü���ȼ���ܹ�����ˮ�Ͷ�����̼��װ��D�пɼ����棬������ձ��ڱ���ˮ�����֣�CH4+2O2 CO2+2H2O��˵�����۹ܵ����к��м��飬Ҫ�������ɵĶ�����̼����ʹ�ó���ʯ��ˮ������ʯ��ˮ����Ǽ���֤�������˶�����̼��
CO2+2H2O��˵�����۹ܵ����к��м��飬Ҫ�������ɵĶ�����̼����ʹ�ó���ʯ��ˮ������ʯ��ˮ����Ǽ���֤�������˶�����̼��
��4�����ݷ�Ӧ�Ļ�ѧ����ʽCH4+2O2 CO2+2H2O�������ļ���������ȫȼ������ˮ�������̼��������=��2��18����44=9��11��������۹ܵ�������CO��ȼ���������ж�����̼������������Ӧ��
CO2+2H2O�������ļ���������ȫȼ������ˮ�������̼��������=��2��18����44=9��11��������۹ܵ�������CO��ȼ���������ж�����̼������������Ӧ��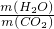 ��9��11��
��9��11��
�ʴ�Ϊ��
��1��H2S
��2��CO2��CO2+Ca��OH��2�TCaCO3��+H2O
��3��CH4��CH4+2O2 CO2+2H2O������ʯ��ˮ
CO2+2H2O������ʯ��ˮ
��4��9��11
�������������CO�Ļ�ԭ�ԣ�CO2��ʹ����ʯ��ˮ����ǡ�CH4ȼ�����ɶ�����̼��ˮ�����ʣ������Ŀ�е���Ϣ�����۹ܵ��еĴ��л�����һ�������·��ͻ����CO��CO2��H2S��CH4�Ⱥ�H2S��������CuSO4��Һ��Ӧ���ɺ�ɫ�������ٹ۲�ͼ��ʵ�鲽������ͭ��Һ���������̼��B����ʯ��ˮ���������̼��C����������Һ���տ����еĶ�����̼�����ɿ��ٽ���⣮
������������Ϣ��ʵ��̽���⣬ֻ�������������ʵ����ʲ���ȷ˳���ĵó���ȷ�𰸣�
��1��װ��A��������ͭ��Һ���������ⷴӦ���ɺ�ɫ�����������ֺ�ɫ����֤�����۹ܵ��������⣬��ѧʽΪH2S��
��2��װ��B���dz����ʯ��ˮ��������̼��ʹ������ǣ���Bװ�ó��ְ�ɫ���ǣ�˵�����۹ܵ�����������̼����Ӧ��ѧ����ʽΪCO2+Ca��OH��2�TCaCO3��+H2O��
��3�����ü���ȼ���ܹ�����ˮ�Ͷ�����̼��װ��D�пɼ����棬������ձ��ڱ���ˮ�����֣�CH4+2O2
 CO2+2H2O��˵�����۹ܵ����к��м��飬Ҫ�������ɵĶ�����̼����ʹ�ó���ʯ��ˮ������ʯ��ˮ����Ǽ���֤�������˶�����̼��
CO2+2H2O��˵�����۹ܵ����к��м��飬Ҫ�������ɵĶ�����̼����ʹ�ó���ʯ��ˮ������ʯ��ˮ����Ǽ���֤�������˶�����̼����4�����ݷ�Ӧ�Ļ�ѧ����ʽCH4+2O2
 CO2+2H2O�������ļ���������ȫȼ������ˮ�������̼��������=��2��18����44=9��11��������۹ܵ�������CO��ȼ���������ж�����̼������������Ӧ��
CO2+2H2O�������ļ���������ȫȼ������ˮ�������̼��������=��2��18����44=9��11��������۹ܵ�������CO��ȼ���������ж�����̼������������Ӧ��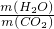 ��9��11��
��9��11���ʴ�Ϊ��
��1��H2S
��2��CO2��CO2+Ca��OH��2�TCaCO3��+H2O
��3��CH4��CH4+2O2
 CO2+2H2O������ʯ��ˮ
CO2+2H2O������ʯ��ˮ��4��9��11
�������������CO�Ļ�ԭ�ԣ�CO2��ʹ����ʯ��ˮ����ǡ�CH4ȼ�����ɶ�����̼��ˮ�����ʣ������Ŀ�е���Ϣ�����۹ܵ��еĴ��л�����һ�������·��ͻ����CO��CO2��H2S��CH4�Ⱥ�H2S��������CuSO4��Һ��Ӧ���ɺ�ɫ�������ٹ۲�ͼ��ʵ�鲽������ͭ��Һ���������̼��B����ʯ��ˮ���������̼��C����������Һ���տ����еĶ�����̼�����ɿ��ٽ���⣮
������������Ϣ��ʵ��̽���⣬ֻ�������������ʵ����ʲ���ȷ˳���ĵó���ȷ�𰸣�

��ϰ��ϵ�д�
�����Ŀ

 <____________����ԭ��Ʒ���к���CO������ԭ��Ʒ���оͲ���CO���밴��˼·���������������ա�
<____________����ԭ��Ʒ���к���CO������ԭ��Ʒ���оͲ���CO���밴��˼·���������������ա�