��Ŀ����
����Ŀ����ͼ��������ʵ��������ȡ����ʱ���õIJ���������
��1��ʵ��������ϡHCl��ʯ��ʯ��ȡ������̼��
�ٷ�Ӧԭ�����û�ѧ����ʽ��ʾΪ_____��
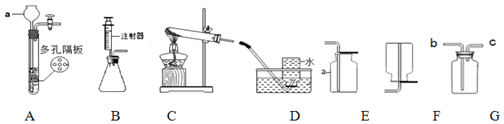
��������ˮ���ռ�CO2��������������A��D��H��L��M��N��O��_____��
������73g 10%��ϡ���ᣬ��20%��������Һ������Ϊ_____�����Ƶ���Ҫ���������ǣ����㣬_____�����ܡ�
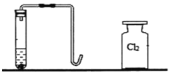
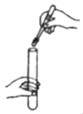
��2��С��ʹ����ͼ��ʾװ�ã���ʵ�������ù���������Һ�Ͷ���������ȡ������
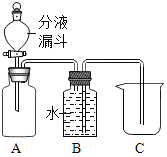
��ʵ��ǰ��С�������Һ©���м���ˮ����Һ©���Ļ���ʹˮ����Aƿ�У�ͬʱ�۲�Bƿ�ڴ��̵��ܿ��Ƿ������ݳ��֡�������������Ŀ����_____��
��С����2.5gMnO2����Aƿ�У���ͨ����Һ©�������м���100g����������Һ����Ӧ��ȫ���ռ���1.6g��������Aƿ�е�ʣ������ˡ�ϴ�Ӳ��������������������Ϊ_____�����ù���������Һ�����ʵ���������Ϊ_____��
���𰸡�CaCO3+2HCl�TCaCl2+H2O+CO2�� C 36.5g ���� ���װ�������� 2.5g 3.4%
��������
��1����ʯ��ʯ����Ҫ�ɷ���̼��ƣ�̼��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧԭ�����û�ѧ����ʽ��ʾΪ��CaCO3+2HCl�TCaCl2+H2O+CO2����
��������ˮ���ռ�CO2��������������A��D��H��L��M��N��O��C��
������73g 10%��ϡ���ᣬ��20%��������Һ������Ϊ��73g��10%��20%��36.5g�����Ƶ���Ҫ���������ǣ����㣬���������ܣ�
��2����ʵ��ǰ��С�������Һ©���м���ˮ����Һ©���Ļ���ʹˮ����Aƿ�У�ͬʱ�۲�Bƿ�ڴ��Ķ̵��ܿ��Ƿ������ݳ��֣�������������Ŀ���Ǽ��װ�������ԣ�
�ڶ��������Ǵ�������Ӧǰ���������䣬��Aƿ�е�ʣ������ˡ�ϴ�Ӳ��������������������Ϊ2.5g��
�裺������������Ϊx��
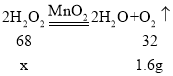
![]() x��3.4g��
x��3.4g��
���ù���������Һ�����ʵ���������Ϊ��![]() ��
��

 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д�����Ŀ������֮����ڽ��桢���л������ϵ�����йظ���֮���������ͼ��ʾ�Ĺ�ϵ��������ѡ���еĸ������ͼ����ʾ��ϵ����( )
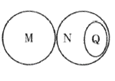
A | B | C | D | |
M | ������ | ������ | �����仯 | �ֽⷴӦ |
N | ������ | ���� | ��ѧ�仯 | ȼ�� |
Q | ���� | ������ | �������� | ���Ϸ�Ӧ |
A. AB. BC. CD. D