��Ŀ����
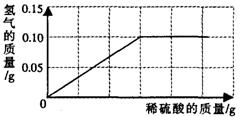
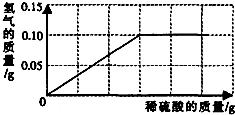 �����Ͼ����в����̷��Ի�ͭð��ƽ�Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�С�������о���ѧϰС���ȡ����Ʒ10g����������μ���9.8%��ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ�����������������Һ��������ϵ��ͼ��ʾ��
�����Ͼ����в����̷��Ի�ͭð��ƽ�Ϊ�˲ⶨ��ͭ��ͭ��п�Ͻ𣩵���ɣ�С�������о���ѧϰС���ȡ����Ʒ10g����������μ���9.8%��ϡ�������պò��ٲ�������Ϊֹ����Ӧ���������ɵ�����������������Һ��������ϵ��ͼ��ʾ���Լ��㣺�����ռ���������һλС����
��1����Ʒ��ͭ������������
��2����Ӧ��������Һ�����ʵ�����������
��3��ͨ������о����㷢���˼����ͭ��ƽ�ķ�����
�μ�ϡ���ᣬ���Ƿ������ݲ���
�μ�ϡ���ᣬ���Ƿ������ݲ���
����������1������п�����ᷴӦ�Ļ�ѧ����ʽ����ͼ��������ɵ���������0.10g�����Լ�����μӷ�Ӧ��п�������������������Ʒ��ͭ��������������2������п�����ᷴӦ�Ļ�ѧ����ʽ����ͼ��������ɵ���������0.10g�����Լ������������п�������������������������п��Һ����������������
��3���ƽ��ܺ�ϡ���ᷴӦ������ͭ�е�п���Ժ�ϡ���ᷴӦ�����Ծݴ˽����⣮
��3���ƽ��ܺ�ϡ���ᷴӦ������ͭ�е�п���Ժ�ϡ���ᷴӦ�����Ծݴ˽����⣮
����⣺��ͼ����Ϣ����֪����������������Ϊ0.1g
��п����Ϊx����������п������Ϊy��
Zn+H2SO4�TZnSO4+H2��
65 161 2
x y 0.1g
=
=
��ã�x=3.25g y=8.05g
��1������ͭ��ͭ����������Ϊ
��100%=67.5%
��2����Ӧ��������Һ�����ʵ���������Ϊ��
��100%=15.1%
��3���ƽ��ܺ�ϡ���ᷴӦ����ͭ�е�п���Ժ�ϡ���ᷴӦ�������ͭ��ƽ�ķ������μ�ϡ���ᣬ���Ƿ������ݲ�����
�𣺣�1������ͭ��ͭ����������Ϊ67.5%��
��2����Ӧ��������Һ�����ʵ���������Ϊ15.1%��
��3���μ�ϡ���ᣬ���Ƿ������ݲ�����
��п����Ϊx����������п������Ϊy��
Zn+H2SO4�TZnSO4+H2��
65 161 2
x y 0.1g
| 65 |
| x |
| 161 |
| y |
| 2 |
| 0.1g |
��ã�x=3.25g y=8.05g
��1������ͭ��ͭ����������Ϊ
| 10g-3.25g |
| 10g |
��2����Ӧ��������Һ�����ʵ���������Ϊ��
| 8.05g |
| 3.25g+50g-0.1g |
��3���ƽ��ܺ�ϡ���ᷴӦ����ͭ�е�п���Ժ�ϡ���ᷴӦ�������ͭ��ƽ�ķ������μ�ϡ���ᣬ���Ƿ������ݲ�����
�𣺣�1������ͭ��ͭ����������Ϊ67.5%��
��2����Ӧ��������Һ�����ʵ���������Ϊ15.1%��
��3���μ�ϡ���ᣬ���Ƿ������ݲ�����
������Ҫ�����������Ŀ�����ȣ�Ҫ�������Ǹ��ݻ�ѧ��Ӧ����ʽ�ļ��㲽���ʽ���Լ���֮��ص�֪ʶ�ȣ�Ȼ���������������龰ϸ�µط������⣬��ϸ�ĵ�̽��������������ĿҪ����������ѡ����ɣ�

��ϰ��ϵ�д�
 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ
 �ⶨ��ͭ(ͭ��п�Ͻ�)����ɣ�С�������о���
�ⶨ��ͭ(ͭ��п�Ͻ�)����ɣ�С�������о���