��Ŀ����
�����С��һ�����ʵ������ȡ������̼��̽����
��һ��ѡ��ҩƷ��С��������ҩƷ�������о���ʵ���¼���£�
����ʵ��٢ڣ�����̽����ͬ�������ض���ȡ������̼�������Ӱ�죻
����ʵ��٢ۣ�����̽��______����ȡ������̼�������Ӱ�죻
С��ѡ��ڢ���ҩƷ����ȡ������̼������ҩƷ������Ӧ�Ļ�ѧ����ʽΪ______����ѡ��ڢ���ҩƷ��ԭ����______��
������ѡ��װ�ã�
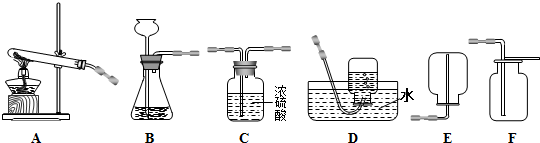
��1��д��ͼ�д�������������ƣ���______����______��
��2��С��ѡ�����巢�����ռ���װ��Ϊ______������A��E��ѡ������������______��
��3�������ɵ�����ͨ��ʯ����Һ�У���Һ��죬����д����Ӧ�Ļ�ѧ����ʽ��______��
��4��װ��B��Ȼ������㣬�������Ʒ�Ӧ���ʣ����ͼ2��ѡȡ______������ţ�ȡ��B�еĵ��������Դﵽ���Ʒ�Ӧ���ʵ�Ŀ�ģ����濪��ص����ã�
��һ��ѡ��ҩƷ��С��������ҩƷ�������о���ʵ���¼���£�
| ��� | ҩƷ | ʵ������ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ��ĩ״ʯ��ʯ��ϡ���� | �����������ʺܿ� |
����ʵ��٢ۣ�����̽��______����ȡ������̼�������Ӱ�죻
С��ѡ��ڢ���ҩƷ����ȡ������̼������ҩƷ������Ӧ�Ļ�ѧ����ʽΪ______����ѡ��ڢ���ҩƷ��ԭ����______��
������ѡ��װ�ã�

��1��д��ͼ�д�������������ƣ���______����______��
��2��С��ѡ�����巢�����ռ���װ��Ϊ______������A��E��ѡ������������______��
��3�������ɵ�����ͨ��ʯ����Һ�У���Һ��죬����д����Ӧ�Ļ�ѧ����ʽ��______��
��4��װ��B��Ȼ������㣬�������Ʒ�Ӧ���ʣ����ͼ2��ѡȡ______������ţ�ȡ��B�еĵ��������Դﵽ���Ʒ�Ӧ���ʵ�Ŀ�ģ����濪��ص����ã�
��һ���Ա�ʵ�����Ʒ�����ֻ��������һ�ֱ���������ʵ��٢�ֻ�м�����ͬ������ʵ��٢�ֻ��ʯ��ʯ����״��ͬ����ѡ��ڢ���ҩƷ��ԭ����̼��������ᷴӦ���������������ˮ��������ʯ��ʯ���棬�����ʯ��ʯ����������Ӵ���ʹ��Ӧ�ٶȼ�����ֹͣ��
��������1����dz������������ƽ���⣻
��2����ȡ������̼��̼��ƺ����ᣬ����Ҫ���ȣ����Է���װ��ѡ��B�����ڶ�����̼������ˮ����������ˮ���ռ����ܶȱȿ�����ֻ���������ſ������ռ������ռ�װ��ѡ��C�����������ǽ�ȼ�ŵ�ľ���ŵ�����ƿ�ڣ��ܹ�Ϩ��˵��������
��3����ʹʯ����Һ�������岻һ���Ƕ�����̼����֤������̼�ó���ʯ��ˮ��
��4���ӿ��Ʒ�Ӧ���ʺ��濪����������濼�ǣ�Ӧ��ѡ���ע�����ģ�ע�����ɿ��Ʒ�Ӧ�ٶȣ�
�ʴ�Ϊ����һ����ͬ���ʯ��ʯ��״��CaCO3+2HCl�TCO2��+H2O+CaCl2����Ӧ���������������ˮ��������ʯ��ʯ���棬ʹ��Ӧ�ٶȼ�����ֹͣ��
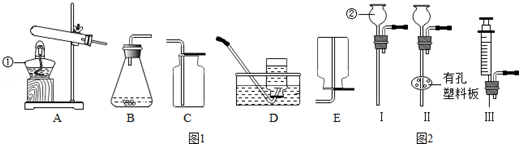
��������1���پƾ��Ƣڳ���©����
��2��B��C��ȼ��ľ���쵽ƿ��Ϩ��
��3��CO2+H2O�TH2CO3��
��4����
��������1����dz������������ƽ���⣻
��2����ȡ������̼��̼��ƺ����ᣬ����Ҫ���ȣ����Է���װ��ѡ��B�����ڶ�����̼������ˮ����������ˮ���ռ����ܶȱȿ�����ֻ���������ſ������ռ������ռ�װ��ѡ��C�����������ǽ�ȼ�ŵ�ľ���ŵ�����ƿ�ڣ��ܹ�Ϩ��˵��������
��3����ʹʯ����Һ�������岻һ���Ƕ�����̼����֤������̼�ó���ʯ��ˮ��
��4���ӿ��Ʒ�Ӧ���ʺ��濪����������濼�ǣ�Ӧ��ѡ���ע�����ģ�ע�����ɿ��Ʒ�Ӧ�ٶȣ�
�ʴ�Ϊ����һ����ͬ���ʯ��ʯ��״��CaCO3+2HCl�TCO2��+H2O+CaCl2����Ӧ���������������ˮ��������ʯ��ʯ���棬ʹ��Ӧ�ٶȼ�����ֹͣ��
��������1���پƾ��Ƣڳ���©����
��2��B��C��ȼ��ľ���쵽ƿ��Ϩ��
��3��CO2+H2O�TH2CO3��
��4����

��ϰ��ϵ�д�
�����Ŀ