��Ŀ����
 ij�������������Ļ��������[��NH4��2SO4]����������أ����Ʒ��װ�ϱ����������[��NH4��2SO4]��70%��Ϊ�ⶨ�û��ʲ�Ʒ������淋�������������������ʵ�飺ȡһ�����ĸû������200g��Һ��ƽ���ֳ����ݣ����������еķ�Ӧ������ȫ��Ӧ��
ij�������������Ļ��������[��NH4��2SO4]����������أ����Ʒ��װ�ϱ����������[��NH4��2SO4]��70%��Ϊ�ⶨ�û��ʲ�Ʒ������淋�������������������ʵ�飺ȡһ�����ĸû������200g��Һ��ƽ���ֳ����ݣ����������еķ�Ӧ������ȫ��Ӧ����ʵ��1������1����Һ�м�����KOH��Һ�����ȣ��ռ�������������m1�ͣ�NH4��2SO4��������ϵ��������ͼ��ʾ��
��ʵ�� 2������2����Һ�������μ����ᱵ��Һ�����ɵij����ͼ�������ᱵ��Һ�Ĺ�ϵ���±�
| �������ᱵ��Һ����/g | 10 | 20 | 45 | 55 |
| ������ɫ��������/g | 2��33 | 4��66 | 6��99 | 6��99 |
��ʵ��1���з�����Ӧ�Ļ�ѧ����ʽΪ
[ʵ��1]�е�1�ݻ�����Һ������淋�����Ϊ
��1������[ʵ��1]��[ʵ��2]�й����ݣ������2�ݻ�����Һ������ص�����
��2���û���������淋���������Ϊ
������ʵ��1�еĻ�ѧ��Ӧ����������������صķ�Ӧ����κͼӦ��������Ӧ���Ρ�ˮ�Ͱ�����
��ʵ��1������ͼ�п��Կ�������狀��������ط�Ӧ���������ɰ���������Ϊ0.68g����Ӧ������淋�������2.64g�����ó���һ�ݻ�����Һ������淋�������
����ʵ��2��������Һ�м������ᱵʱ�����ᱵ�������李������ͬʱ��Ӧ�������ᱵ�������ӱ����п��Է��֣����ɵij���һ����6.99g�����ݻ�ѧ����ʽ��Ҫ֪������ص�����������Ҫ֪������غ����ᱵ��Ӧ���ɶ��ٳ�����Ҫ֪����������ɶ��ٳ������ֱ���֪������炙����ɶ��ٳ��������ʵ��һ�����ݿ�֪��Һ������淋������������������刺������ɶ��ٳ������ݴ˵���ȥ���㣬�Ϳ�֪������ص�������
����������淋���������=
��100%��Ȼ������Ŀ�е�������Ƚϣ����ɵó���Ʒ�ϸ����
��ʵ��1������ͼ�п��Կ�������狀��������ط�Ӧ���������ɰ���������Ϊ0.68g����Ӧ������淋�������2.64g�����ó���һ�ݻ�����Һ������淋�������
����ʵ��2��������Һ�м������ᱵʱ�����ᱵ�������李������ͬʱ��Ӧ�������ᱵ�������ӱ����п��Է��֣����ɵij���һ����6.99g�����ݻ�ѧ����ʽ��Ҫ֪������ص�����������Ҫ֪������غ����ᱵ��Ӧ���ɶ��ٳ�����Ҫ֪����������ɶ��ٳ������ֱ���֪������炙����ɶ��ٳ��������ʵ��һ�����ݿ�֪��Һ������淋������������������刺������ɶ��ٳ������ݴ˵���ȥ���㣬�Ϳ�֪������ص�������
����������淋���������=
| ����淋����� |
| ���������+��������� |
����⣺ʵ��1�з����ķ�Ӧ����������������صķ�Ӧ���仯ѧ��Ӧ����ʽΪ����NH4��2SO4+2KOH�TK2SO4+2NH3��+2H2O��
������ͼ�п��Կ�������һ����Һ������淋�����Ϊ2.64g��
��1�����裺����������ᱵ��Ӧ�������ᱵ����������Ϊx����Һ������ص�����Ϊy
��NH4��2SO4+Ba��NO3��2�T2NH4NO3+BaSO4��
132 233
2.64g x
=
x=4.66g
������������ᱵ��Ӧ���ɳ���������Ϊ6.99g-4.66g=2.33g
K2SO4+Ba��NO3��2�T2KNO3+BaSO4��
174 233
y 2.33g
=
y=1.74g
�û���������淋���������Ϊ
��100%=60.3%
��60.3%��70%
��ò�Ʒ���ϸ�
�ʴ�Ϊ����NH4��2SO4+2KOH�TK2SO4+2NH3��+2H2O
2.64g
��1����1.74g
��2����60.3%�����ϸ�
������ͼ�п��Կ�������һ����Һ������淋�����Ϊ2.64g��
��1�����裺����������ᱵ��Ӧ�������ᱵ����������Ϊx����Һ������ص�����Ϊy
��NH4��2SO4+Ba��NO3��2�T2NH4NO3+BaSO4��
132 233
2.64g x
| 132 |
| 233 |
| 2.64g |
| x |
x=4.66g
������������ᱵ��Ӧ���ɳ���������Ϊ6.99g-4.66g=2.33g
K2SO4+Ba��NO3��2�T2KNO3+BaSO4��
174 233
y 2.33g
| 174 |
| 233 |
| y |
| 2.33g |
y=1.74g
�û���������淋���������Ϊ
| 2.64g |
| 2.64g+1.74g |
��60.3%��70%
��ò�Ʒ���ϸ�
�ʴ�Ϊ����NH4��2SO4+2KOH�TK2SO4+2NH3��+2H2O
2.64g
��1����1.74g
��2����60.3%�����ϸ�
����������Ŀ���ѵ���������ڶ�����Һ������ص����������������Ƶķ�����
������Һ�ľ�һ���ص㣬��һ����Һ�е�����������ڶ�����Һ�����ʵ���������ͬ�ģ�
������Һ�ľ�һ���ص㣬��һ����Һ�е�����������ڶ�����Һ�����ʵ���������ͬ�ģ�

��ϰ��ϵ�д�
 ����������������ϵ�д�
����������������ϵ�д�
�����Ŀ
 ij�������������Ļ��������[��NH4��2SO4]����������أ����Ʒ��װ�ϱ����������[��NH4��2SO4]��70%��Ϊ�ⶨ�û��ʲ�Ʒ������淋�������������������ʵ�飺ȡһ�����ĸû������200g��Һ��ƽ���ֳ����ݣ����������еķ�Ӧ������ȫ��Ӧ��
ij�������������Ļ��������[��NH4��2SO4]����������أ����Ʒ��װ�ϱ����������[��NH4��2SO4]��70%��Ϊ�ⶨ�û��ʲ�Ʒ������淋�������������������ʵ�飺ȡһ�����ĸû������200g��Һ��ƽ���ֳ����ݣ����������еķ�Ӧ������ȫ��Ӧ��
��ʵ��1������1����Һ�м�����KOH��Һ�����ȣ��ռ�������������m1�ͣ�NH4��2SO4��������ϵ��������ͼ��ʾ��
��ʵ�� 2������2����Һ�������μ����ᱵ��Һ�����ɵij����ͼ�������ᱵ��Һ�Ĺ�ϵ���±�
| �������ᱵ��Һ����/g | 10 | 20 | 45 | 55 |
| ������ɫ��������/g | 2��33 | 4��66 | 6��99 | 6��99 |
��ʵ��1���з�����Ӧ�Ļ�ѧ����ʽΪ______��
[ʵ��1]�е�1�ݻ�����Һ������淋�����Ϊ______g��
��1������[ʵ��1]��[ʵ��2]�й����ݣ������2�ݻ�����Һ������ص�����______��
��2���û���������淋���������Ϊ______����______����ϸ�ϸ���Ʒ��
ij�������������Ļ��������[��NH4��2SO4]����������أ����Ʒ��װ�ϱ����������[��NH4��2SO4]��70%��Ϊ�ⶨ�û��ʲ�Ʒ������淋�������������������ʵ�飺ȡһ�����ĸû������200g��Һ��ƽ���ֳ����ݣ����������еķ�Ӧ������ȫ��Ӧ��
��ʵ��1������1����Һ�м�����KOH��Һ�����ȣ��ռ�������������m1�ͣ�NH4��2SO4��������ϵ��������ͼ��ʾ��
��ʵ�� 2������2����Һ�������μ����ᱵ��Һ�����ɵij����ͼ�������ᱵ��Һ�Ĺ�ϵ���±�
�ش��������⣺
��ʵ��1���з�����Ӧ�Ļ�ѧ����ʽΪ______��
[ʵ��1]�е�1�ݻ�����Һ������淋�����Ϊ______g��
��1������[ʵ��1]��[ʵ��2]�й����ݣ������2�ݻ�����Һ������ص�����______��
��2���û���������淋���������Ϊ______����______����ϸ�ϸ���Ʒ��
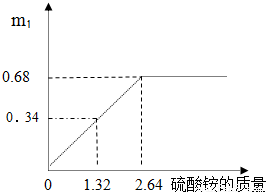
��ʵ��1������1����Һ�м�����KOH��Һ�����ȣ��ռ�������������m1�ͣ�NH4��2SO4��������ϵ��������ͼ��ʾ��
��ʵ�� 2������2����Һ�������μ����ᱵ��Һ�����ɵij����ͼ�������ᱵ��Һ�Ĺ�ϵ���±�
| �������ᱵ��Һ����/g | 10 | 20 | 45 | 55 |
| ������ɫ��������/g | 2��33 | 4��66 | 6��99 | 6��99 |
��ʵ��1���з�����Ӧ�Ļ�ѧ����ʽΪ______��
[ʵ��1]�е�1�ݻ�����Һ������淋�����Ϊ______g��
��1������[ʵ��1]��[ʵ��2]�й����ݣ������2�ݻ�����Һ������ص�����______��
��2���û���������淋���������Ϊ______����______����ϸ�ϸ���Ʒ��
