��Ŀ����
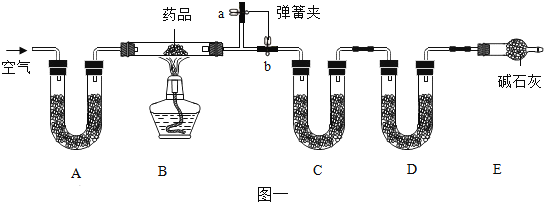
����Ŀ����ͼ��ʵ���ҳ��õ�ʵ��������װ�ã��ش��������⡣
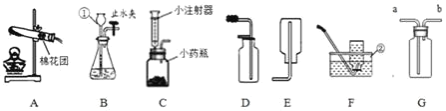
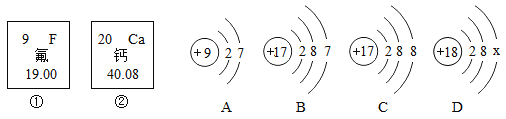
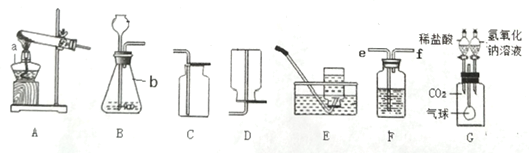
��1��ʵ�����ø��������ȡ���ռ�һƿ��Ϊ���������������������Ϊ����װ����ţ�_____����Ӧ�Ļ�ѧ����ʽΪ_____��
��2��ʵ���ҳ���װ�� B ��ȡ������̼���䷴Ӧ�Ļ�ѧ����ʽΪ_____��ijͬѧ���������е���Ʒ���ͼ C װ�ã���װ���� B ������ŵ���_____��Сע�����������൱��ʵ�����е�_____�����������ƣ���
��3���ر� B װ���е�ֹˮ�кӳ���©������ƿ��ע��һ������ˮ����ֹ����ͼ��ʾ���� B װ��_____������©����������©����������ȷ��������
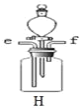
��4��ʵ���ҳ��ô����ƹ���ͼ�ʯ�ҹ����ϼ����Ʊ����飨CH4�����壬����װ��Ӧѡ��_____������ţ�����֪�����ܶȱȿ���С������ G װ�����ռ�������Ӧ��_____��a �� b��ͨ�롣
��5��������̼��ˮ��Һ��ʹ��ɫʯ����ֽ��죬����װ�� H ֤��ʹ��ɫʯ����ֽ����������̼�������ˮ�������̼��Ӧ�ò�ȡ��ʵ�����˳����_____������ĸ���ţ���
�ٴ� f ��ͨ����
�ڴӷ�Һ©���еμ�����ˮ
�۴� e ��ͨ������̼
�ܽ��������ɫʯ����ֽ���� H װ�õĹ��ƿ��
A �ܢۢ٢ڢ� B �ۢܢڢ٢� C �ܢڢۢ٢�
���𰸡�AF ![]()
![]() �ܹ����Ʒ�Ӧ���� ��Һ©�� ��©�� A b A
�ܹ����Ʒ�Ӧ���� ��Һ©�� ��©�� A b A
��������
��1���ø��������ȡ���ռ�һƿ��Ϊ��������������Ӧװ��Ӧѡ�������ȷ���װ�ã���Ϊ����������ˮ����������ˮ���ռ���������Ϊ����������ѡ��AF����ѧ����ʽΪ��![]()
��2��ʵ���ҳ���װ�� B ��ȡ������̼���䷴Ӧ�Ļ�ѧ����ʽΪ��![]() ��Cװ����Bװ������ŵ�����ܹ����Ʒ�Ӧ���ʣ�Сע�����൱��ʵ�����еķ�Һ©����
��Cװ����Bװ������ŵ�����ܹ����Ʒ�Ӧ���ʣ�Сע�����൱��ʵ�����еķ�Һ©����
��3���ر� B װ���е�ֹˮ�кӳ���©������ƿ��ע��һ������ˮ����ֹ��©����Һ�治�½���˵�����������ã���©����
��4��ʵ���ҳ��ô����ƹ���ͼ�ʯ�ҹ����ϼ����Ʊ����飨CH4�����壬����װ��Ӧѡ��A������Gװ�����ռ����飬��Ϊ�����ܶȱȿ���С�����Դ�bͨ�룬������a���ų���
��5��������̼��ˮ��Һ��ʹ��ɫʯ����ֽ��죬����װ�� H ֤��ʹ��ɫʯ����ֽ����������̼�������ˮ�������̼�����Ƚ��������ɫʯ����ֽ���� H װ�õĹ��ƿ�У�Ȼ���e��ͨ������̼������ɫ˵��������̼����ʹ��ɫʯ����ֽ��죬���Ŵ�f��ͨ��������װ���ڵĶ�����̼�Ÿɾ����ٴӷ�Һ©���еμ�����ˮ������ɫ˵��ˮ����ʹ��ɫʯ����ֽ��죬����ٴ�e��ͨ������̼�����˵���Ƕ�����̼��ˮ��Һʹ��ɫʯ����ֽ��졣��ѡA��

 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�