��Ŀ����
�������ʾ��ͼ������������ʶ��ѧ���ʺ����⻯ѧ��Ӧ����1������
 ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ��� ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ��� ��ʾ
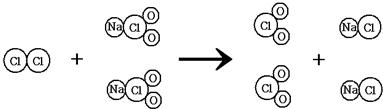
��ʾ��2��ClO2����һ������ˮ�����������ҹ�����ɹ����Ƴ���ȡClO2���·������䷴Ӧ
���۹�����ͼ��ʾ��
����д���÷�Ӧ�Ļ�ѧ����ʽ
���������������У�������������ǣ��ѧʽ����ͬ��
����ClO2�к���Ԫ�ص���������Ϊ
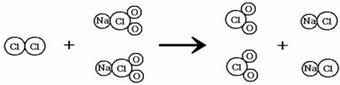
��������1������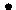 ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ��� ��ʾ��ԭ�ӣ����֪
��ʾ��ԭ�ӣ����֪ ��ʾ�ķ��ţ�
��ʾ�ķ��ţ�
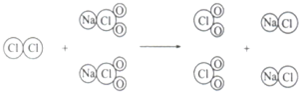
��2�����ɷ�Ӧ���۹���ͼ������д���÷�Ӧ�Ļ�ѧ����ʽ��
�ڸ���������ĸ������֪������������������������������ʣ���֪��Ԫ�صĻ��ϼ�Ϊ+3�۵����ʣ�
����ClO2�к���Ԫ�ص���������ΪΪ��Ԫ�ص����ԭ��������ClO2����Է���������
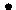 ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ��� ��ʾ��ԭ�ӣ����֪
��ʾ��ԭ�ӣ����֪ ��ʾ�ķ��ţ�
��ʾ�ķ��ţ���2�����ɷ�Ӧ���۹���ͼ������д���÷�Ӧ�Ļ�ѧ����ʽ��
�ڸ���������ĸ������֪������������������������������ʣ���֪��Ԫ�صĻ��ϼ�Ϊ+3�۵����ʣ�
����ClO2�к���Ԫ�ص���������ΪΪ��Ԫ�ص����ԭ��������ClO2����Է���������
����⣺
��1������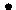 ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ��� ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ��� ��ʾH2O��
��ʾH2O��
��2�����ɷ�Ӧ���۹���ͼ������д���÷�Ӧ�Ļ�ѧ����ʽCl2+2NaClO2�T2ClO2+2NaCl��
�������������Ԫ����ɣ�����һ��Ϊ��Ԫ�أ����������������У��������������ClO2����֪��Ԫ�صĻ��ϼ�Ϊ-2�ۣ���Ԫ�صĻ��ϼ�Ϊ+1�ۣ�����Ԫ�صĻ��ϼ�Ϊ+3�۵�������NaClO2��
����ClO2�к���Ԫ�ص���������Ϊ
��100%=47.4%
�ʴ�Ϊ����1��H2O��
��2����Cl2+2NaClO2�T2ClO2+2NaCl��
��ClO2�� NaClO2��
��47.4%
��1������
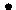 ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ��� ��ʾ��ԭ�ӣ���
��ʾ��ԭ�ӣ��� ��ʾH2O��
��ʾH2O����2�����ɷ�Ӧ���۹���ͼ������д���÷�Ӧ�Ļ�ѧ����ʽCl2+2NaClO2�T2ClO2+2NaCl��
�������������Ԫ����ɣ�����һ��Ϊ��Ԫ�أ����������������У��������������ClO2����֪��Ԫ�صĻ��ϼ�Ϊ-2�ۣ���Ԫ�صĻ��ϼ�Ϊ+1�ۣ�����Ԫ�صĻ��ϼ�Ϊ+3�۵�������NaClO2��
����ClO2�к���Ԫ�ص���������Ϊ
| 35.5 |
| 67.5 |
�ʴ�Ϊ����1��H2O��
��2����Cl2+2NaClO2�T2ClO2+2NaCl��
��ClO2�� NaClO2��
��47.4%
������ͨ����������ģ�ͣ�����ѧ���Ĺ۲������ͶԻ����������������������������Ԫ�����������ļ��㷽����

��ϰ��ϵ�д�
 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
�����Ŀ
 ��ʾ
��ʾ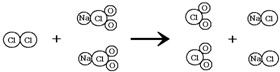
 ��ʾ
��ʾ