��Ŀ����
 ˮ������ͨ�����������֮һ��
ˮ������ͨ�����������֮һ����1����ˮ���кܶ��֣����С�ˮ�����ڴ�������� ������ĸ��ţ���
A����ˮ B������ˮ C����Ȫˮ D������ˮ��
��2�����ˮ��֤��ˮ���⡢������Ԫ����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��ˮ����Ҫ���ܼ��ͻ���ԭ�ϣ��ȼҵ�Ա���ʳ��ˮΪԭ�ϻ���ռ�Ȼ�����Ʒ����Ӧԭ��Ϊ��2NaCl+2H2O
 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2������20��ʱ��NaCl���ܽ����36g�����¶��£�����ʳ��ˮ���������ܼ���������Ϊ ��
���ռ�����ڴ�������й©����Ӧ�Ļ�ѧ����ʽΪ ��
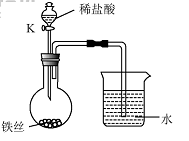
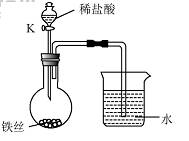
��4��ˮ�ڻ�ѧʵ���о�����Ҫ�����ã�����˿���ڳ�ʪ�Ŀ����У���ͼ��ʾ����һ��ʱ��۲쵽������Һ���½�����K���μ�ϡ���ᣬ�۲쵽������Һ���½������ܿ�������ð�����ر�K������͵�����Һ���������½���ԭ�� ��
���𰸡���������1�����ݺ�ˮ������ˮ����Ȫˮ�������ʶ�����ˮ�в��������ʽ��н��
��2������ˮͨ���ֽܷ������������������н��
��3�������ܽ�ȵĺ����Լ��ռ�����ᷴӦ���������ƺ�ˮ���н��
��4���������ڳ�ʪ�Ŀ����������Լ����������Ӧ�����������н��
����⣺��1����ˮ������ˮ����Ȫˮ�������ʣ����ڻ���������ˮ�в��������ʣ����ڴ�������D��
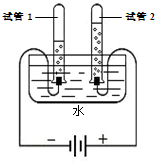
��2��ˮͨ���ֽܷ������������������÷�Ӧ�Ļ�ѧ����ʽΪ��2H2O 2H2��+O2�������2H2O
2H2��+O2�������2H2O 2H2��+O2����
2H2��+O2����
��3����20��ʱ��NaCl���ܽ����36g����ָ���¶���100gˮ������ܽ�36g�Ȼ��ƣ����Ա���ʳ��ˮ���������ܼ���������=36��100=9��25�����9��25��
���ռ�����ڴ�������й©�����ռ�����ᷴӦ���������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2NaOH+H2SO4�TNa2SO4+2H2O�����2NaOH+H2SO4�TNa2SO4+2H2O��
��4�����ڳ�ʪ�Ŀ��������⣬���Ŀ����е�������ʹƿ��ѹǿ��С�����Թ۲쵽������Һ�����������������Ӧ��������������ʹƿ��ѹǿ��۲쵽������Һ���½������ܿ�������ð����������ڳ�ʪ�Ŀ��������⣬���Ŀ����е�������ʹƿ��ѹǿ��С�����Թ۲쵽������Һ�����������������Ӧ��������������ʹƿ��ѹǿ��۲쵽������Һ���½������ܿ�������ð����
���������⿼�����ÿα�֪ʶ����������������ܼ���ѧ���Կα�֪ʶ�����⣬ѵ��ѧ����˼ά������
��2������ˮͨ���ֽܷ������������������н��
��3�������ܽ�ȵĺ����Լ��ռ�����ᷴӦ���������ƺ�ˮ���н��
��4���������ڳ�ʪ�Ŀ����������Լ����������Ӧ�����������н��
����⣺��1����ˮ������ˮ����Ȫˮ�������ʣ����ڻ���������ˮ�в��������ʣ����ڴ�������D��
��2��ˮͨ���ֽܷ������������������÷�Ӧ�Ļ�ѧ����ʽΪ��2H2O
 2H2��+O2�������2H2O
2H2��+O2�������2H2O 2H2��+O2����
2H2��+O2������3����20��ʱ��NaCl���ܽ����36g����ָ���¶���100gˮ������ܽ�36g�Ȼ��ƣ����Ա���ʳ��ˮ���������ܼ���������=36��100=9��25�����9��25��
���ռ�����ڴ�������й©�����ռ�����ᷴӦ���������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2NaOH+H2SO4�TNa2SO4+2H2O�����2NaOH+H2SO4�TNa2SO4+2H2O��
��4�����ڳ�ʪ�Ŀ��������⣬���Ŀ����е�������ʹƿ��ѹǿ��С�����Թ۲쵽������Һ�����������������Ӧ��������������ʹƿ��ѹǿ��۲쵽������Һ���½������ܿ�������ð����������ڳ�ʪ�Ŀ��������⣬���Ŀ����е�������ʹƿ��ѹǿ��С�����Թ۲쵽������Һ�����������������Ӧ��������������ʹƿ��ѹǿ��۲쵽������Һ���½������ܿ�������ð����
���������⿼�����ÿα�֪ʶ����������������ܼ���ѧ���Կα�֪ʶ�����⣬ѵ��ѧ����˼ά������

��ϰ��ϵ�д�
�����Ŀ
ˮ������ͨ�����������֮һ��
��1����ˮ���кܶ��֡����С�ˮ�����ڴ�������� ������ĸ��ţ���
| A����ˮ | B������ˮ | C����Ȫˮ | D������ˮ |
��2�����ˮ��֤��ˮ���⡢������Ԫ����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��ˮ����Ҫ���ܼ��ͻ���ԭ�ϡ��ȼҵ�Ա���ʳ��ˮΪԭ�ϻ���ռ�Ȼ�����Ʒ����Ӧԭ��Ϊ�� 2NaCl+2H2O 2NaOH+H2��
2NaOH+H2��
��20��ʱ��NaCl ���ܽ����36 g�����¶��£�����ʳ��ˮ���������ܼ���������Ϊ ��
���ռ�����ڴ�������й©����Ӧ�Ļ�ѧ����ʽΪ ��
��4��ˮ�ڻ�ѧʵ���о�����Ҫ���á�����˿���ڳ�ʪ�Ŀ����У���ͼ��ʾ����һ��ʱ��۲쵽������Һ���½������ܿ�������ð�����ر� K������͵�����Һ���������½���ԭ�� ��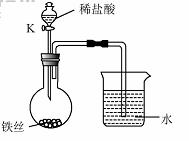
ˮ������ͨ�����������֮һ��
��1����ˮ���кܶ��֡����С�ˮ�����ڴ�������� ������ĸ��ţ���
| A����ˮ | B������ˮ | C����Ȫˮ | D������ˮ |
��3��ˮ����Ҫ���ܼ��ͻ���ԭ�ϡ��ȼҵ�Ա���ʳ��ˮΪԭ�ϻ���ռ�Ȼ�����Ʒ����Ӧԭ��Ϊ�� 2NaCl+2H2O
 2NaOH+H2��
2NaOH+H2�� ��20��ʱ��NaCl ���ܽ����36 g�����¶��£�����ʳ��ˮ���������ܼ���������Ϊ ��
���ռ�����ڴ�������й©����Ӧ�Ļ�ѧ����ʽΪ ��
��4��ˮ�ڻ�ѧʵ���о�����Ҫ���á�����˿���ڳ�ʪ�Ŀ����У���ͼ��ʾ����һ��ʱ��۲쵽������Һ���½������ܿ�������ð�����ر� K������͵�����Һ���������½���ԭ�� ��
 ˮ������ͨ�����������֮һ��
ˮ������ͨ�����������֮һ�� 2NaOH+H2��
2NaOH+H2�� 
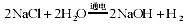 ��
��  Ϊ ��
Ϊ ��