��Ŀ����
����ͬѧȡ��һ�鳤ʱ������ڿ����е�����Ʒ������Ϊ15.6g��������Ʒ����50gϡ�����У�����Ʒ��ȫ�ܽ⣬�����ݲ������õ�ֻ��һ�����ʵ���Һ65g��
��1���������巢���ķ�Ӧ�Ļ�ѧ����ʽ��______��
��2�����ݲ�������������г��������Ʒ��ʣ�����������ı���ʽ��______��
��3������ϡ���������ʵ���������Ϊ��______��
��4����Ӧ����Һ�м���35gˮ����ˮ����Һ�����ʵ���������Ϊ��______��
��5����Ҫʵ������ϡ����______g������21.9%������100g��
�⣺��1���������������ᷴӦ�������������壬����������ķ�Ӧ����������ķ�Ӧ������ʽΪ��2Al+6HCl=2AlCl3+3H2����
��2���������غ㶨�ɿ�֪��Ӧ�������ļ���ֵ��Ϊ��������������������15.6g+50g-65g=0.6g��
������������x ��Ӧ���Ȼ����������y
2Al+6HCl=2AlCl3+3H2��
54 219 6
x y 0.6g
���ݲ�������������г��������Ʒ��ʣ�����������ı���ʽΪ�� ��x=5.4g������������������15.6g-5.4g=10.2g
��x=5.4g������������������15.6g-5.4g=10.2g
���� ��y=21.9g
��y=21.9g
��3��������������Ӧ���Ȼ���������z
Al2O3+6HCl=2AlCl3+3H2O��
102 219
10.2g z
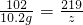 ����z=21.9g�����뷴Ӧ����Һ��ֻ����һ�����ʣ���50g����Ҳ��ǡ�÷�Ӧ����
����z=21.9g�����뷴Ӧ����Һ��ֻ����һ�����ʣ���50g����Ҳ��ǡ�÷�Ӧ���� =87.6%
=87.6%
��3�������Ȼ����е���Ԫ�غ��Ȼ����е���Ԫ��������ȣ�
���Ȼ�����������w������w�� =��21.9g+21.9g����
=��21.9g+21.9g����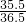 ��w=53.4g��������Ӧ����Һ�м���35gˮ����ˮ����Һ�����ʵ���������Ϊ
��w=53.4g��������Ӧ����Һ�м���35gˮ����ˮ����Һ�����ʵ���������Ϊ ��100%=53.4%��
��100%=53.4%��
��4������Ҫ�������������n
n��87.6%=100g��21.9%
n=25g
�ʴ�Ϊ����1��2Al+6HCl=2AlCl3+3H2������2�� ����3��87.6%����4��53.4%����5��25��
����3��87.6%����4��53.4%����5��25��
��������1���������ܹ������ᷴӦ���������ķ���ʽ��ɣ�
��2�����ݷ�Ӧǰ������ʵ�����֮��Ϊ������������ɣ�
��3��������������������Ӧ���Ȼ������������������������������
��4������ϡ��ǰ�����ʵ��������������ɣ�
��5������������Һʱ���ʵ�������ȷ������
������������Ҫ�����йغ��������ʵĻ�ѧ����ʽ������������������ļ��㣬�ѶȽϴ�
��2���������غ㶨�ɿ�֪��Ӧ�������ļ���ֵ��Ϊ��������������������15.6g+50g-65g=0.6g��
������������x ��Ӧ���Ȼ����������y
2Al+6HCl=2AlCl3+3H2��
54 219 6
x y 0.6g
���ݲ�������������г��������Ʒ��ʣ�����������ı���ʽΪ��
 ��x=5.4g������������������15.6g-5.4g=10.2g
��x=5.4g������������������15.6g-5.4g=10.2g����
 ��y=21.9g
��y=21.9g��3��������������Ӧ���Ȼ���������z
Al2O3+6HCl=2AlCl3+3H2O��
102 219
10.2g z
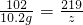 ����z=21.9g�����뷴Ӧ����Һ��ֻ����һ�����ʣ���50g����Ҳ��ǡ�÷�Ӧ����
����z=21.9g�����뷴Ӧ����Һ��ֻ����һ�����ʣ���50g����Ҳ��ǡ�÷�Ӧ���� =87.6%
=87.6%��3�������Ȼ����е���Ԫ�غ��Ȼ����е���Ԫ��������ȣ�
���Ȼ�����������w������w��
 =��21.9g+21.9g����
=��21.9g+21.9g����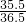 ��w=53.4g��������Ӧ����Һ�м���35gˮ����ˮ����Һ�����ʵ���������Ϊ
��w=53.4g��������Ӧ����Һ�м���35gˮ����ˮ����Һ�����ʵ���������Ϊ ��100%=53.4%��
��100%=53.4%����4������Ҫ�������������n
n��87.6%=100g��21.9%
n=25g
�ʴ�Ϊ����1��2Al+6HCl=2AlCl3+3H2������2��
 ����3��87.6%����4��53.4%����5��25��
����3��87.6%����4��53.4%����5��25����������1���������ܹ������ᷴӦ���������ķ���ʽ��ɣ�
��2�����ݷ�Ӧǰ������ʵ�����֮��Ϊ������������ɣ�
��3��������������������Ӧ���Ȼ������������������������������
��4������ϡ��ǰ�����ʵ��������������ɣ�
��5������������Һʱ���ʵ�������ȷ������
������������Ҫ�����йغ��������ʵĻ�ѧ����ʽ������������������ļ��㣬�ѶȽϴ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ