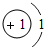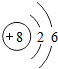��Ŀ����
��2012?��ƽ��ģ�⣩�������ǻ�����������������֯����Ҫԭ�ϣ�����ͨ��ʳ���õĵ�������θ������ˮ��Ӧ���������ɰ����ᱻ�������գ������ᣨ��ѧʽΪC3H7O2N���������е�һ�֣���ش��������⣺
��1�������������C��H��O��N��ԭ�ӵĸ�����Ϊ
��2�����������Է���������
��3���ϸ��̷�ÿ100g�к�������Լ18g���������е�Ԫ�ص�ƽ����������Ϊ16%����ÿ100g�ϸ��̷��У���Ԫ������Ϊ
��1�������������C��H��O��N��ԭ�ӵĸ�����Ϊ
3��7��2��1
3��7��2��1
����2�����������Է���������
89
89
����Ԫ�ص���������Ϊ15.73%
15.73%
����������ȷ��0.01%������3���ϸ��̷�ÿ100g�к�������Լ18g���������е�Ԫ�ص�ƽ����������Ϊ16%����ÿ100g�ϸ��̷��У���Ԫ������Ϊ
2.88
2.88
g���ֲⶨij�̷�ÿ100g�к��е�Ԫ�ص�����Ϊ2g���������̷��������ϸ�
���ϸ�
����ϸ��ϸ��̷ۣ���������1�����ݱ�����Ļ�ѧʽ��֪��������C��H��O��N ��ԭ�ӵĸ����ȣ�
��2�����ݱ��������Է����������Ǹ�ԭ�ӵ����ԭ������֮���Լ��������е�Ԫ�ص���������Ϊ��
��100%����
��3�����ݵ����ʵ��������������е�Ԫ�ص�ƽ����������=�̷��е�Ԫ�ص��������㼴�ɣ�
��2�����ݱ��������Է����������Ǹ�ԭ�ӵ����ԭ������֮���Լ��������е�Ԫ�ص���������Ϊ��
| �������ԭ����������ԭ�Ӹ��� |
| ���������Է������� |
��3�����ݵ����ʵ��������������е�Ԫ�ص�ƽ����������=�̷��е�Ԫ�ص��������㼴�ɣ�
����⣺��1���ɱ����ữѧʽΪC3H7O2N��֪����������C��H��O��N ��ԭ�ӵĸ�����=3��7��2��1��
��2�����������Է�������=12��3+1��7+16��2+14=89���������к��е�Ԫ�ص���������Ϊ��
��100%=15.73%��
��3��ÿ100g�ϸ��̷��У���Ԫ������=18g��16%=2.88g����Ϊ2g��2.88g���������̷������ϸ��̷ۣ�
�ʴ�Ϊ����1��3��7��2��1����2��89��15.73%����3��2.88�����ϸ�
��2�����������Է�������=12��3+1��7+16��2+14=89���������к��е�Ԫ�ص���������Ϊ��
| 14 |
| 89 |
��3��ÿ100g�ϸ��̷��У���Ԫ������=18g��16%=2.88g����Ϊ2g��2.88g���������̷������ϸ��̷ۣ�
�ʴ�Ϊ����1��3��7��2��1����2��89��15.73%����3��2.88�����ϸ�
������������Ҫ����ѧ��������ѧ��ѧ֪ʶ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ
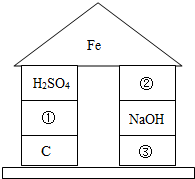 ��2012?��ƽ��ģ�⣩ijѧϰС���ͬѧ�ԸǷ��ӵ���Ϸ������������Ҫ����֮�����ϵ����ͼ����Ϸ�������ϡ������ڵ����ʼ���ɷ�����Ӧ��
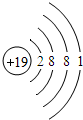
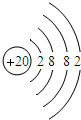
��2012?��ƽ��ģ�⣩ijѧϰС���ͬѧ�ԸǷ��ӵ���Ϸ������������Ҫ����֮�����ϵ����ͼ����Ϸ�������ϡ������ڵ����ʼ���ɷ�����Ӧ�� ��2012?��ƽ��ģ�⣩Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ���ͼ��Ԫ�����ڱ��е�һ������Ӹ�ͼ��ȡ����Ϣ�У���ȷ���ǣ�������
��2012?��ƽ��ģ�⣩Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ���ͼ��Ԫ�����ڱ��е�һ������Ӹ�ͼ��ȡ����Ϣ�У���ȷ���ǣ�������