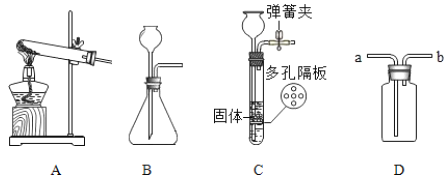
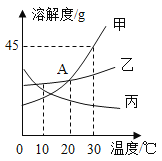
��Ŀ����
��ѧ������ϢϢ��أ�����ѧ�û�ѧ֪ʶ�������ǽ��һЩ���š�
��1������ʱ���е��Ͳ����Ż𣬿��ù��Ǹ��������ԭ��Ϊ______��
��2����������������Ӧ����㷺��һ�ֽ�������Ԫ����ؿ��к�������Ԫ����ɻ�����Ļ�ѧʽΪ______��д��һ�ּ��ɣ���
��3�����϶��ĺš�����������״�����������������ʵ�飬����ѡ���������Ͳˡ������ȣ������Ͳ���Ϊ�����ṩ��Ӫ������Ҫ��______����ˮ�⣩��
��4����ʯȼ�ϵĴ���ʹ�ã������˻�������Ⱦ����Դ�Ŀݽߵ����⣬���������ڹ㷺�ƹ�ʹ���Ҵ����ͣ���ô���______��
 ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д�
ѧ�����νӽ̲��Ͼ���ѧ������ϵ�д� Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�������֪�գ���֪������һ�ֳ����Ļ�ѧ˼ά��������������ʵ������֪ʶ���Ͳ���ȷ���ǣ�������
ѡ�� | ��ʵ | ���� |
A | ˮ���������в�ͬ�Ļ�ѧ���� | �������ʵķ��Ӳ�ͬ |
B | ��Ȼ���е�ˮ����̬�仯 | ˮ���ӵĴ�С���¶ȸı���ı� |
C | ��Ȼ���У����ʯ��Ӳ����ʯī���� | ̼ԭ�����з�ʽ��ͬ |
D | �ᡢ�����Һ���ܵ��� | ��Һ���������ƶ������� |
A.A B.B C.C D.D
ij��ѧ��ȤС���ͬѧ��ѧϰ���꼶����ѧ���²��е����Ͽ�Ƭ��ʯ�������ʯ���γɡ�ʱ������������ˮ��̼��Ƶ��������ж�����̼��ˮʱ���ᷴӦ�����ܽ��Խϴ��̼����ƣ�CaCO3+CO2+H2O�TCa(HCO3)2�����뵽ʵ�����г���ʯ��ˮ�������̼��Ӧ������̼��ƣ�Ca(OH)2+CO2�TCaCO3��+H2O���Գ�ʱ�������Һ��ͨ��CO2����Ӧ����Һ�е�������ɲ�����Ũ�����Ȥ��
��������⣩һ����CO2��NaOH��Һ��Ӧ������������ʲô��
���������ϣ�
��1��ͨ������CO2��Ӧ�Ļ�ѧ����ʽΪ______��
��2��ͨ�����CO2����Ӧ�Ļ�ѧ����ʽΪNa2CO3+CO2+H2O�T2NaHCO3��
��3��̼�����ζ��ǿ�����ˮ�ģ�BaCO3������ˮ��
��4��̼��������Һ�ʼ��ԡ�
��������룩
��1������ΪNaOH��Na2CO3��
��2������ΪNa2CO3��
��3������Ϊ______���ѧʽ����
��4������ΪNaHCO3��
�����ʵ�飩
ʵ�鲽�� | ʵ������ | ʵ����� |
��1���ò�����պȡ��Ӧ����Һ������pH��ֽ�� | pH=9 | ����Һ�� ______ �� |
��2��ȡ��Ӧ����Һ�������Թ��У������еμӹ�����BaCl2��Һ | �� ______ ���� | ���루4�������� |
��3��ȡ���裨2���е��ϲ���Һ������ϡ���� | ������ð�� | ���� ______ ������ |
���ó����ۣ����루3��������
�����۽�����
��1����ͬѧ�����ʵ�鲽�裨1���Ƕ���ġ�����Ϊ��ʵ������Ƿ���Ҫ��______�����Ҫ������Ҫ������
��2��ͬѧ����һ�ΰ�Ŀ��Ͷ���˽̲ģ��������Ȼ�ѹǿ��Сʱ��Ca(HCO3)2�TCaCO3��+CO2��+H2O����������ɷ����������NaHCO3���ķ�Ӧԭ����֮���ƣ���д��NaHCO3���ȷֽ�Ļ�ѧ����ʽ��______ ��
����˼Ӧ�ã������ʯ��ˮ�в���ͨ�������̼���۲쵽��������______��