��Ŀ����
Ϊ�ⶨH2SO4��CuSO4�Ļ����Һ�����ʵĺ�����Сǿ��С����Ʋ�����������ʵ�飮���������ϡ���Cu��OH��2���������ֽ����CuO��H2O����BaSO4���������ѷֽ⣮
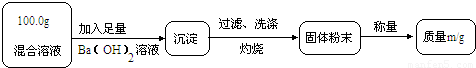
��ʵ��һ��Ŀ�ģ��ⶨ100.0g�����Һ��CuSO4������
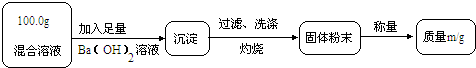
��1��Сǿ��Ϊm1��CuO��������С��������ɣ���Ϊm1��
��2��Ϊ�õ�CuO��������С��������Ba��OH��2��Һ������һ����Һ������Һ������
A��NaOH��Һ��B��KOH��Һ��C��BaCl2��Һ
��3���Ľ�ʵ���С���õ�8.0g CuO������100.0g�����Һ��CuSO4��������
�⣺
��ʵ�����Ŀ�ģ��ⶨ100.0 g�����Һ��H2SO4����������
��Ҫʵ�鲽�����£�
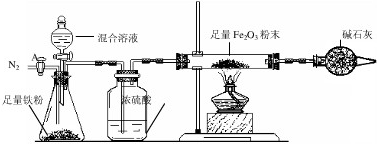
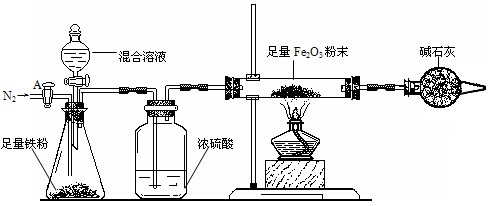
a����ͼ��װ��������������ԣ�װ��ҩƷ������Fe2O3������Ϊm2g��
b������A��ͨ��N2һ��ʱ�䣬�رջ���A��
c����ȼ�ƾ��ƣ���μ���100.0g�����Һ��
d���ⶨ��Ӧ������Ӳ�ʲ����������ʵ���������������ʾ����
e������Ӧ������Ϩ��ƾ��ƣ�����A������ͨһ���N2��
��¼����
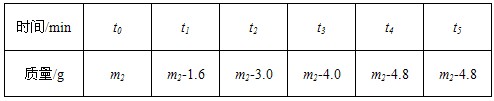
| ʱ��/min | t0 | t1 | t2 | t3 | t4 | t5 |
| ����/g | m2 | m2-1.6 | m2-3.0 | m2-4.0 | m2-4.8 | m2-4.8 |
��4����������ʵ�����ݣ�������Fe2O3��Ӧ���������ĺ��ԣ�������100.0g�����Һ��H2SO4����������Ϊ
��������1����Ϊ����������Һ��H2SO4��CuSO4��Ӧ������Cu��OH��2��BaSO4��Cu��OH��2���������ֽ����CuO��H2O��BaSO4���������ѷֽ⣮�ݴ˴��⣻
��2�����������֪��Cu��OH��2���������ֽ����CuO��H2O�����������Һ�ͻ����Һ��Ӧ��������������ͭ���ɣ�
��3���ٸ��ݼ���������ͭ�Ļ�ѧ����ʽ������ͭ���������г�����ʽ�����ɼ������Ӧ����������Cu��OH��2����������
�ڸ�������ͭ���������Ʒ�Ӧ�Ļ�ѧ����ʽ�Լ����м�����ķ�Ӧ����������Cu��OH��2�������������ɼ����100.0g�����Һ�к�CuSO4������
��4����ͼ����֪����ȫ��Ӧʱ���Թ��������仯Ϊ4.8g����ʵ����ʧȥ����Ԫ�ص�������Ҳ���ǹ��������仯����Ȼ�����Fe2O3��H2��Ӧ�Ļ�ѧ����ʽ���ɼ���� H2 ������������������������ʵ�������Ȼ���������������ʽ���㼴�ɣ�
��2�����������֪��Cu��OH��2���������ֽ����CuO��H2O�����������Һ�ͻ����Һ��Ӧ��������������ͭ���ɣ�
��3���ٸ��ݼ���������ͭ�Ļ�ѧ����ʽ������ͭ���������г�����ʽ�����ɼ������Ӧ����������Cu��OH��2����������
�ڸ�������ͭ���������Ʒ�Ӧ�Ļ�ѧ����ʽ�Լ����м�����ķ�Ӧ����������Cu��OH��2�������������ɼ����100.0g�����Һ�к�CuSO4������
��4����ͼ����֪����ȫ��Ӧʱ���Թ��������仯Ϊ4.8g����ʵ����ʧȥ����Ԫ�ص�������Ҳ���ǹ��������仯����Ȼ�����Fe2O3��H2��Ӧ�Ļ�ѧ����ʽ���ɼ���� H2 ������������������������ʵ�������Ȼ���������������ʽ���㼴�ɣ�
����⣻��1����Ϊ����������Һ��H2SO4��CuSO4��Ӧ������Cu��OH��2��BaSO4������֪������֪��Cu��OH��2���������ֽ����CuO��H2O��BaSO4���������ѷֽ⣬�ʿ��ж�m1ΪCuO��BaSO4��������
��ѡCuO��BaSO4��
��2����ΪA��B��Һ�ж���OH���ӣ�����������ͭ��Ӧ����������ͭ��Cu��OH��2���������ֽ����CuO��H2O�����Եõ�CuO��������
��ѡAB��
��3���⣺���跴Ӧ����������Cu��OH��2��������Ϊx��������ã�
Cu��OH��2
CuO+H2O��
98 80
x 8.0g
=
��
��֮�ã�x=9.8g��
����100.0g�����Һ�к�CuSO4����Ϊy��
CuSO4+2NaOH=Cu��OH��2��+Na2SO4
160 98
y 9.8g
��
=
��
��֮�ã�y=16.0g��
��100.0g�����Һ�к�CuSO4����Ϊ16.0g��
��4������������Ӧ������������Ϊx��
Fe2O3+3H2
2Fe+3H2O��m
6 160-112=48
x 4.8g
��
=
��֮�ã�x=0.6g��
������0.6g������Ҫ�����������Ϊ0.6g�£�
��100%��=29.4g
��������Һ����������Ϊ
��100%=29.4%
�ʴ�Ϊ��29.4%��
��ѡCuO��BaSO4��
��2����ΪA��B��Һ�ж���OH���ӣ�����������ͭ��Ӧ����������ͭ��Cu��OH��2���������ֽ����CuO��H2O�����Եõ�CuO��������
��ѡAB��
��3���⣺���跴Ӧ����������Cu��OH��2��������Ϊx��������ã�
Cu��OH��2
| ||
98 80
x 8.0g
| 98 |
| 80 |
| x |
| 8.0g |
��֮�ã�x=9.8g��
����100.0g�����Һ�к�CuSO4����Ϊy��
CuSO4+2NaOH=Cu��OH��2��+Na2SO4
160 98
y 9.8g
��
| 160 |
| 98 |
| y |
| 9.8g |
��֮�ã�y=16.0g��
��100.0g�����Һ�к�CuSO4����Ϊ16.0g��
��4������������Ӧ������������Ϊx��
Fe2O3+3H2
| ||
6 160-112=48
x 4.8g
��
| 6 |
| x |
| 48 |
| 4.8g |
��֮�ã�x=0.6g��
������0.6g������Ҫ�����������Ϊ0.6g�£�
| 2 |
| 98 |
��������Һ����������Ϊ
| 29.4g |
| 100g |
�ʴ�Ϊ��29.4%��
������������Ҫ����ѧ�����û�ѧ����ʽ������������ʽ���м����������

��ϰ��ϵ�д�
�����Ŀ
Ϊ�ⶨH2SO4��CuSO4�Ļ����Һ�����ʵĺ�����Сǿ��С����Ʋ�����������ʵ�飮
���������ϡ���Cu��OH��2���������ֽ����CuO��H2O����BaSO4���������ѷֽ⣮
��ʵ��һ��Ŀ�ģ��ⶨ100.0g�����Һ��CuSO4������

��1��Сǿ��Ϊm1��CuO��������С��������ɣ���Ϊm1��________��������
��2��Ϊ�õ�CuO��������С��������Ba��OH��2��Һ������һ����Һ������Һ������________������ĸ����
A��NaOH��Һ��B��KOH��Һ��C��BaCl2��Һ
��3���Ľ�ʵ���С���õ�8.0g CuO������100.0g�����Һ��CuSO4��������
�⣺
��ʵ�����Ŀ�ģ��ⶨ100.0 g�����Һ��H2SO4����������
��Ҫʵ�鲽�����£�
a����ͼ��װ��������������ԣ�װ��ҩƷ������Fe2O3������Ϊm2g��
b������A��ͨ��N2һ��ʱ�䣬�رջ���A��
c����ȼ�ƾ��ƣ���μ���100.0g�����Һ��
d���ⶨ��Ӧ������Ӳ�ʲ����������ʵ���������������ʾ����
e������Ӧ������Ϩ��ƾ��ƣ�����A������ͨһ���N2��
��¼����
| ʱ��/min | t0 | t1 | t2 | t3 | t4 | t5 |
| ����/g | m2 | m2-1.6 | m2-3.0 | m2-4.0 | m2-4.8 | m2-4.8 |
��4����������ʵ�����ݣ�������Fe2O3��Ӧ���������ĺ��ԣ�������100.0g�����Һ��H2SO4����������Ϊ________���������õ�����Է���������Fe2O3-160��H2SO4-98��
��2008?��ɽ��Ϊ�ⶨH2SO4��CuSO4�Ļ����Һ�����ʵĺ�����Сǿ��С����Ʋ�����������ʵ�飮
���������ϡ���Cu��OH��2���������ֽ����CuO��H2O����BaSO4���������ѷֽ⣮
��ʵ��һ��Ŀ�ģ��ⶨ100.0g�����Һ��CuSO4������
��1��Сǿ��Ϊm1��CuO��������С��������ɣ���Ϊm1��______��������
��2��Ϊ�õ�CuO��������С��������Ba��OH��2��Һ������һ����Һ������Һ������______������ĸ����
A��NaOH��Һ��B��KOH��Һ��C��BaCl2��Һ
��3���Ľ�ʵ���С���õ�8.0g CuO������100.0g�����Һ��CuSO4��������
�⣺
��ʵ�����Ŀ�ģ��ⶨ100.0 g�����Һ��H2SO4����������
��Ҫʵ�鲽�����£�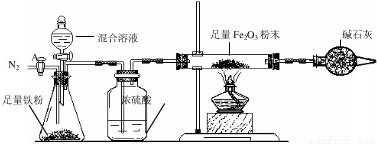
a����ͼ��װ��������������ԣ�װ��ҩƷ������Fe2O3������Ϊm2g��
b������A��ͨ��N2һ��ʱ�䣬�رջ���A��
c����ȼ�ƾ��ƣ���μ���100.0g�����Һ��
d���ⶨ��Ӧ������Ӳ�ʲ����������ʵ���������������ʾ����
e������Ӧ������Ϩ��ƾ��ƣ�����A������ͨһ���N2��
��¼����
���ˡ�ϴ�Ӹ������
��4����������ʵ�����ݣ�������Fe2O3��Ӧ���������ĺ��ԣ�������100.0g�����Һ��H2SO4����������Ϊ______���������õ�����Է���������Fe2O3-160��H2SO4-98��
���������ϡ���Cu��OH��2���������ֽ����CuO��H2O����BaSO4���������ѷֽ⣮
��ʵ��һ��Ŀ�ģ��ⶨ100.0g�����Һ��CuSO4������

��1��Сǿ��Ϊm1��CuO��������С��������ɣ���Ϊm1��______��������
��2��Ϊ�õ�CuO��������С��������Ba��OH��2��Һ������һ����Һ������Һ������______������ĸ����
A��NaOH��Һ��B��KOH��Һ��C��BaCl2��Һ
��3���Ľ�ʵ���С���õ�8.0g CuO������100.0g�����Һ��CuSO4��������
�⣺
��ʵ�����Ŀ�ģ��ⶨ100.0 g�����Һ��H2SO4����������
��Ҫʵ�鲽�����£�

a����ͼ��װ��������������ԣ�װ��ҩƷ������Fe2O3������Ϊm2g��
b������A��ͨ��N2һ��ʱ�䣬�رջ���A��
c����ȼ�ƾ��ƣ���μ���100.0g�����Һ��
d���ⶨ��Ӧ������Ӳ�ʲ����������ʵ���������������ʾ����
e������Ӧ������Ϩ��ƾ��ƣ�����A������ͨһ���N2��
��¼����
| ʱ��/min | t | t1 | t2 | t3 | t4 | t5 |
| ����/g | m2 | m2-1.6 | m2-3.0 | m2-4.0 | m2-4.8 | m2-4.8 |
��4����������ʵ�����ݣ�������Fe2O3��Ӧ���������ĺ��ԣ�������100.0g�����Һ��H2SO4����������Ϊ______���������õ�����Է���������Fe2O3-160��H2SO4-98��
28����2008?��ɽ��Ϊ�ⶨH2SO4��CuSO4�Ļ����Һ�����ʵĺ�����Сǿ��С����Ʋ�����������ʵ�飮
���������ϡ���Cu��OH��2���������ֽ����CuO��H2O����BaSO4���������ѷֽ⣮
��ʵ��һ��Ŀ�ģ��ⶨ100.0g�����Һ��CuSO4������
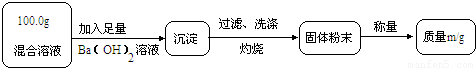
��1��Сǿ��Ϊm1��CuO��������С��������ɣ���Ϊm1��______��������
��2��Ϊ�õ�CuO��������С��������Ba��OH��2��Һ������һ����Һ������Һ������______������ĸ����
A��NaOH��Һ��B��KOH��Һ��C��BaCl2��Һ
��3���Ľ�ʵ���С���õ�8.0g CuO������100.0g�����Һ��CuSO4��������
�⣺
��ʵ�����Ŀ�ģ��ⶨ100.0 g�����Һ��H2SO4����������
��Ҫʵ�鲽�����£�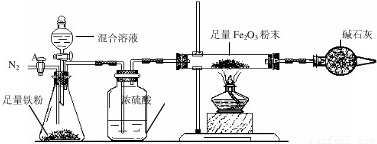
a����ͼ��װ��������������ԣ�װ��ҩƷ������Fe2O3������Ϊm2g��
b������A��ͨ��N2һ��ʱ�䣬�رջ���A��
c����ȼ�ƾ��ƣ���μ���100.0g�����Һ��
d���ⶨ��Ӧ������Ӳ�ʲ����������ʵ���������������ʾ����
e������Ӧ������Ϩ��ƾ��ƣ�����A������ͨһ���N2��
��¼����
���ˡ�ϴ�Ӹ������
��4����������ʵ�����ݣ�������Fe2O3��Ӧ���������ĺ��ԣ�������100.0g�����Һ��H2SO4����������Ϊ______���������õ�����Է���������Fe2O3-160��H2SO4-98��
���������ϡ���Cu��OH��2���������ֽ����CuO��H2O����BaSO4���������ѷֽ⣮
��ʵ��һ��Ŀ�ģ��ⶨ100.0g�����Һ��CuSO4������

��1��Сǿ��Ϊm1��CuO��������С��������ɣ���Ϊm1��______��������
��2��Ϊ�õ�CuO��������С��������Ba��OH��2��Һ������һ����Һ������Һ������______������ĸ����
A��NaOH��Һ��B��KOH��Һ��C��BaCl2��Һ
��3���Ľ�ʵ���С���õ�8.0g CuO������100.0g�����Һ��CuSO4��������
�⣺
��ʵ�����Ŀ�ģ��ⶨ100.0 g�����Һ��H2SO4����������
��Ҫʵ�鲽�����£�

a����ͼ��װ��������������ԣ�װ��ҩƷ������Fe2O3������Ϊm2g��
b������A��ͨ��N2һ��ʱ�䣬�رջ���A��
c����ȼ�ƾ��ƣ���μ���100.0g�����Һ��
d���ⶨ��Ӧ������Ӳ�ʲ����������ʵ���������������ʾ����
e������Ӧ������Ϩ��ƾ��ƣ�����A������ͨһ���N2��
��¼����
| ʱ��/min | t | t1 | t2 | t3 | t4 | t5 |
| ����/g | m2 | m2-1.6 | m2-3.0 | m2-4.0 | m2-4.8 | m2-4.8 |
��4����������ʵ�����ݣ�������Fe2O3��Ӧ���������ĺ��ԣ�������100.0g�����Һ��H2SO4����������Ϊ______���������õ�����Է���������Fe2O3-160��H2SO4-98��