ĢāÄæÄŚČŻ
£Ø8·Ö£©Ė®ŹĒÉśĆüÖ®Ō“£¬ČĖĄąµÄČÕ³£Éś»īÓė¹¤Å©ŅµÉś²ś¶¼Ąė²»æŖĖ®”£
£Ø1£©Š”ŗģĄūÓĆĶ¼¢ŁĖłŹ¾µÄ×°ÖĆĢ½¾æĖ®µÄ×é³É”£ĶصēŅ»¶ĪŹ±¼äŗó£¬ŹŌ¹ÜBÖŠĖłŹÕ¼ÆµÄĘųĢåĪŖ £¬øĆŹµŃéĖµĆ÷Ė®ŹĒÓÉ ×é³ÉµÄ”£
¢Ł  ¢Ś
¢Ś ¢Ū
¢Ū
£Ø2£©Óū³żČ„²»ČÜÓŚĖ®µÄÄąÉ³£¬Ó¦²ÉÓƵÄ×°ÖĆČēĶ¼¢ŚĖłŹ¾£¬ĘäÖŠŠ”ĀŃŹÆ”¢ŹÆӢɳŗĶÅņĖÉĆŽµÄ×÷ÓĆŹĒ ”£
£Ø3£©2011Äź11ŌĀ³õ£¬Éń°ĖÓėĢģ¹¬Ņ»ŗŶŌ½Ó³É¹¦±źÖ¾×ÅĪŅ¹śŗ½Ģģ¹¤³ĢĀõ³öĮĖ¹Ų¼üŅ»²½”£·¢Éä”°Éń°Ė”± ĖłÓĆµÄ£ØČēĶ¼¢ŪĖłŹ¾£©µÄČ¼ĮĻŹĒŅŗĒāŗĶŅŗŃõ£¬ĒāĘųČ¼ÉÕŹ±·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_________________________”£
£Ø4£©ÄæĒ°£¬Šķ¶ą×ŌĄ“Ė®³§²ÉÓĆĀČĘų½ųŠŠÉ±¾śĻū¶¾”£³£ĪĀĻĀ£¬ĀČĘųÄÜÓėĖ®·“Ӧɜ³ÉŃĪĖįŗĶ“ĪĀČĖį£ØHClO£©£¬“ĪĀČĖįÄÜɱ¾śĻū¶¾”£ĀČĘųÓėĖ®·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ_____________________”£
£Ø5£©ŗģČȵÄĢśÄÜÓėĖ®ÕōĘų·“Ó¦£¬·Å³öĘųĢåX£¬Ķ¬Ź±Éś³É¹ĢĢåY”£XĘųĢåææ½ü»šŃęµć»šŹ±£¬ÄÜČ¼ÉÕ»ņ·¢³ö±¬ĆłÉł”£Ęä·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ3Fe + 4H2O  4X
+ Y”£ŌņXµÄ»ÆѧŹ½ĪŖ
£¬YµÄ»ÆѧŹ½ĪŖ
ӣ
4X
+ Y”£ŌņXµÄ»ÆѧŹ½ĪŖ
£¬YµÄ»ÆѧŹ½ĪŖ
ӣ
£Ø7·Ö£©
£Ø1£©O2£ØŃõĘų£©£¬ĒāŌŖĖŲŗĶŃõŌŖĖŲ
£Ø2£©¹żĀĖ
£Ø3£©2H2£«O2  2H2O
2H2O
£Ø4£©Cl2+H2O=HCl+HClO £Ø2·Ö£© £Ø5£©Fe3O4 H2
”¾½āĪö”æĀŌ


 Ė®ŹĒÉśĆüÖ®Ō“£¬ČĖĄąµÄČÕ³£Éś»īŗĶ¹¤Å©ŅµÉś²ś¶¼Ąė²»æŖĖ®£®ÓŅĶ¼¼×ŹĒĖ®µē½āŹµŃéµÄ×°ÖĆĶ¼£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
Ė®ŹĒÉśĆüÖ®Ō“£¬ČĖĄąµÄČÕ³£Éś»īŗĶ¹¤Å©ŅµÉś²ś¶¼Ąė²»æŖĖ®£®ÓŅĶ¼¼×ŹĒĖ®µē½āŹµŃéµÄ×°ÖĆĶ¼£¬»Ų“šĻĀĮŠĪŹĢā£ŗ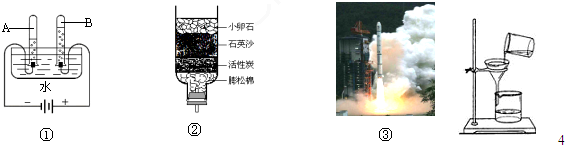
 Ė®ŹĒÉśĆüÖ®Ō“£¬ČĖĄąµÄČÕ³£Éś»īĄė²»æŖĖ®£®
Ė®ŹĒÉśĆüÖ®Ō“£¬ČĖĄąµÄČÕ³£Éś»īĄė²»æŖĖ®£®