��Ŀ����
21����2007?���ݣ�ú��ʯ�ͺ���Ȼ������Ҫ�ġ�������������Դ������ȼ�ղ�������Ķ�����̼�����ʻ�Ի���������ص�Ӱ�죮�ҹ������߶����ӡ����ܼ��š���ȷ���ҹ����ꡰ���绷���ա�������ͱ�ʶ����ͼ�� ����
�� l �����д�ʩ�����ҹ������绷���ա�������ǣ�����ĸ��
A�����Ʒ�չ�ߺ��ܵIJ�ҵ
B���ƹ�ʹ�á��Ҵ����͡��������к������ŷ�
C��Ϊ�˼��ٷ����Ա�������������Ⱦ���������Ӹ��̴�
�� 2 ����Ȼ������Ҫ�ɷ��ǣ�����ȫȼ�յĻ�ѧ����ʽΪ
�� 3 ����ͼ���е���Ϣ������ࣺ������̼�����������ŷţ�ʹ�Ӿ磬����ȫ���ů��Ϊ���ٶ�����̼������ŷŶԻ�����Ӱ�죬��������һ���£���
��ѧ�Ҳ�ȡ�����ת������������������̼��������һ��������ͣ���һ�������·�Ӧ������һ����Ҫ�Ļ���ԭ����ϩ��C2H4����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ
�� 4 ��Ϊ�����Դ��ȱ�ͻ�����Ⱦ�����⣬Ŀǰ�д����������õ�����Դ�У�дһ�֣�
���𰸡����������Ʒ�չ�ߺ��ܵIJ�ҵ���ƹ�ʹ�á��Ҵ����͡��������к������ŷ�����Ч�ؼ���������Ⱦ����Ȼ������Ҫ�ɷ��Ǽ��飬���ݷ�Ӧ��������P�������غ㶨�ɿ�����ȷ����д��ѧ����ʽ��������̼��һ����Ҫ���������壮���������õ�����Դ��̫���ܡ����ܡ����ܡ�ˮ�ܡ������ܵ���Դ��
����⣺��1�����Ʒ�չ�ߺ��ܵIJ�ҵ���ƹ�ʹ�á��Ҵ����͡������ҹ������绷���ա����⣮���AB��
��2����Ȼ������Ҫ�ɷ��Ǽ��飮������飮
����ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH4+2O2 CO2+2H2O��
CO2+2H2O��
��3��������̼��һ����Ҫ���������壬ֲ�����ֿ��Լ�������ЧӦ���������ЧӦ��ֲ�����֣�
������̼��������Ӧ������ϩ��ˮ�Ļ�ѧ����ʽΪ��2CO2+6H2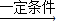 C2H4+4H2O��
C2H4+4H2O��
��4��Ŀǰ�д����������õ�����Դ��̫���ܡ����ܡ����ܡ�ˮ�ܡ������ܵ���Դ��������ܣ�
�����������ʱҪ���ջ�ѧ����ʽ����д����������ܻ�������Ҫ�ԣ�ֻ���������ܶ�����������ȷ���жϣ�
����⣺��1�����Ʒ�չ�ߺ��ܵIJ�ҵ���ƹ�ʹ�á��Ҵ����͡������ҹ������绷���ա����⣮���AB��
��2����Ȼ������Ҫ�ɷ��Ǽ��飮������飮
����ȼ�����ɶ�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH4+2O2
 CO2+2H2O��
CO2+2H2O����3��������̼��һ����Ҫ���������壬ֲ�����ֿ��Լ�������ЧӦ���������ЧӦ��ֲ�����֣�
������̼��������Ӧ������ϩ��ˮ�Ļ�ѧ����ʽΪ��2CO2+6H2
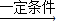 C2H4+4H2O��
C2H4+4H2O����4��Ŀǰ�д����������õ�����Դ��̫���ܡ����ܡ����ܡ�ˮ�ܡ������ܵ���Դ��������ܣ�
�����������ʱҪ���ջ�ѧ����ʽ����д����������ܻ�������Ҫ�ԣ�ֻ���������ܶ�����������ȷ���жϣ�

��ϰ��ϵ�д�
�����Ŀ