��Ŀ����
��2013?˳�����ʼ죩ʵ���ҳ�����ͼ��ʾA��Bװ����ȡ���壬�ش�������⣺
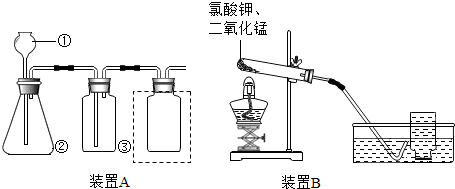
��1��ͼ�Тٵ�����������
��2��С����Aװ����ȡ�ϴ������������������Ӧ�Ļ�ѧ����ʽΪ
��3������װ��Ӧ����
��4��װ��B����ʵ������ȡ��������Ӧ��Ϻ��Ի��ն������̼���ʹ�õ�ԭ����
��5��ʵ���ҳ����Ȼ�粒������ʯ�ҹ��干������ȡ������NH3��������Ϊ����װ��B�Ƿ���У�

��1��ͼ�Тٵ�����������
����©��
����©��
����2��С����Aװ����ȡ�ϴ������������������Ӧ�Ļ�ѧ����ʽΪ
Zn+H2SO4=ZnSO4+H2��
Zn+H2SO4=ZnSO4+H2��
����ƿ����ҪʢװŨ����
Ũ����
�����ڷ����ڽ��ռ�װ���еĵ��ܲ�����������3������װ��Ӧ����
���¶���
���¶���
�������ҵ�˳��װ�������װ�õ������ԣ���4��װ��B����ʵ������ȡ��������Ӧ��Ϻ��Ի��ն������̼���ʹ�õ�ԭ����
���������ڷ�Ӧǰ��������ͻ�ѧ���ʲ��ı�
���������ڷ�Ӧǰ��������ͻ�ѧ���ʲ��ı�
����5��ʵ���ҳ����Ȼ�粒������ʯ�ҹ��干������ȡ������NH3��������Ϊ����װ��B�Ƿ���У�
��
��
����ǡ�����������������������ˮ����������ˮ���ռ�
������������ˮ����������ˮ���ռ�
����������1���ݳ��������ش�
��2��ʵ������ȡ������п��ϡ���ᷴӦ���ݷ�Ӧԭ����д����ʽ��������������Һ������Ũ���ᣬ�������ܶȱȿ���С������Ҫ�Ӷ̵��ܽ�����
��3������װ�õ�˳�������¶��ϣ������ң�
��4��װ��B�����ڼ��ȹ�����ȡ���壬���Թܿ����������ü�������ء����������������ķ�����ȡ�����������������ͻ�ѧ�����ڷ�Ӧǰ�䣻
��5��װ��B�����ڼ��ȹ�����ȡ���壬��ˮ���ռ������岻����ˮ���ݴ˷������
��2��ʵ������ȡ������п��ϡ���ᷴӦ���ݷ�Ӧԭ����д����ʽ��������������Һ������Ũ���ᣬ�������ܶȱȿ���С������Ҫ�Ӷ̵��ܽ�����
��3������װ�õ�˳�������¶��ϣ������ң�
��4��װ��B�����ڼ��ȹ�����ȡ���壬���Թܿ����������ü�������ء����������������ķ�����ȡ�����������������ͻ�ѧ�����ڷ�Ӧǰ�䣻
��5��װ��B�����ڼ��ȹ�����ȡ���壬��ˮ���ռ������岻����ˮ���ݴ˷������
����⣺��1��ͼ�Т��dz���©����
��2��ʵ������ȡ������п��ϡ���ᷴӦ����������п������������ʽ��Zn+H2SO4=ZnSO4+H2����������������Һ������Ũ���ᣬ�������ܶȱȿ���С������Ҫ�Ӷ̵��ܽ�����
��3������װ�õ�˳�������¶��ϣ������ң�
��4��װ��B�����ڼ��ȹ�����ȡ���壬���Թܿ�������˵�����ü�������ء����������������ķ�����ȡ�������������̵������ͻ�ѧ�����ڷ�Ӧǰ�䣬���Կɻ��������ã�
��5�����Ȼ�粒������ʯ�ҹ��干������ȡ������NH3�������ô˷���װ�ã�������������ˮ����������ˮ���ռ������Բ�����װ��B��ȡ������
�ʴ�Ϊ����1������©����
��2��Zn+H2SO4=ZnSO4+H2����Ũ����ռ���������ͼ
��3�����¶��ϣ�
��4�����������ڷ�Ӧǰ��������ͻ�ѧ���ʲ��ı䣻
��5���� ������������ˮ����������ˮ���ռ���
��2��ʵ������ȡ������п��ϡ���ᷴӦ����������п������������ʽ��Zn+H2SO4=ZnSO4+H2����������������Һ������Ũ���ᣬ�������ܶȱȿ���С������Ҫ�Ӷ̵��ܽ�����
��3������װ�õ�˳�������¶��ϣ������ң�
��4��װ��B�����ڼ��ȹ�����ȡ���壬���Թܿ�������˵�����ü�������ء����������������ķ�����ȡ�������������̵������ͻ�ѧ�����ڷ�Ӧǰ�䣬���Կɻ��������ã�
��5�����Ȼ�粒������ʯ�ҹ��干������ȡ������NH3�������ô˷���װ�ã�������������ˮ����������ˮ���ռ������Բ�����װ��B��ȡ������
�ʴ�Ϊ����1������©����

��2��Zn+H2SO4=ZnSO4+H2����Ũ����ռ���������ͼ
��3�����¶��ϣ�
��4�����������ڷ�Ӧǰ��������ͻ�ѧ���ʲ��ı䣻
��5���� ������������ˮ����������ˮ���ռ���
���������⿼����ʵ������ȡ�����ķ�Ӧԭ��������ĸ���ռ���װ�����ӡ�������֪ʶ���������֪ʶ������ȷ���

��ϰ��ϵ�д�
�����Ŀ
 ��2013?˳�����ʼ죩�л�����X�Ĺ�ϵ����ͼ��ʾ����X�������ǣ�������
��2013?˳�����ʼ죩�л�����X�Ĺ�ϵ����ͼ��ʾ����X�������ǣ�������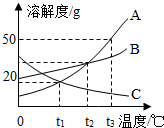 ��2013?˳�����ʼ죩����ͼA��B��C���ֹ�����ܽ�����ش�
��2013?˳�����ʼ죩����ͼA��B��C���ֹ�����ܽ�����ش�