��Ŀ����
 �ձ��ش������ɸ�����һ�˵�վ1�Ż�����������ը����ش��й����⣺
�ձ��ش������ɸ�����һ�˵�վ1�Ż�����������ը����ش��й����⣺��1���ҹ��Ѽ����ձ���й©���ͷŵķ����Ե�131��˵���������ʵ�������
��2���ձ��������ؾ��ŵ�Ƭ���������õ�Ƭ�ɱ������������շ�����Ԫ�ص�131���Ӷ�����
�ټ�״�١�������������ȣ�ݡ������������۹�������
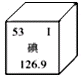
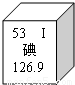
��3��Ԫ�����ڱ��е�Ԫ�ص�ijЩ��Ϣ��ͼ��ʾ������ڵ�131��131Ϊ��ԭ�ӵ����ԭ�����������127��˵����ȷ����
������ͬ��Ԫ�ء�������ԭ����������53������ԭ��������ͬ
��4�������ƻ��˻����ڵ���ȴϵͳ����ȼ�ϰ�����ﯣ�Zr���Ͻ����İ��DZ����ȵ�1200������ʱ�����ˮ������Ӧ��������ﯣ�ZrO2����������������������ȼ�ձ�ը����д������������Ӧ�Ļ�ѧ����ʽ��
��������1���������ӵIJ����˶�������
��2��������ȱ��������Σ��������
��3��ͬ��Ԫ�ص���������ͬ����ԭ���У�������=ԭ������=�˵������
��4�����ݷ�Ӧ�������ͷ�Ӧ������д��ѧ����ʽ��ע����ƽ��������
��2��������ȱ��������Σ��������
��3��ͬ��Ԫ�ص���������ͬ����ԭ���У�������=ԭ������=�˵������
��4�����ݷ�Ӧ�������ͷ�Ӧ������д��ѧ����ʽ��ע����ƽ��������
����⣺��1���ҹ��Ѽ����ձ���й©���ͷŵķ����Ե�131��˵���������ʵ������в����˶������ʣ������Ե�131�������Ѿ��˶����ҹ����ڣ�
��2������ȱ��������Լ�״���ף�Ҳ���Ǵ��Ӳ���
��3����131��131Ϊ��ԭ�ӵ����ԭ�����������127�ĺ˵������ͬ������ͬ��Ԫ�أ���ԭ����������53��
��4��ﯣ�Zr�������ȵ�1200������ʱ�����ˮ������Ӧ��������ﯣ�ZrO2����������������������ȼ�ձ�ը������������Ӧ�Ļ�ѧ����ʽΪ��Zr+2H2O
ZrO2+2H2��2H2+O2
2H2O��ע���ڸ�����ˮ����̬�ģ������ɵ��������治���������ɷ��ţ�
�ʴ�Ϊ����1�������˶���
��2���٣�
��3���٢ڣ�
��4��Zr+2H2O
ZrO2+2H2��2H2+O2
2H2O��
��2������ȱ��������Լ�״���ף�Ҳ���Ǵ��Ӳ���
��3����131��131Ϊ��ԭ�ӵ����ԭ�����������127�ĺ˵������ͬ������ͬ��Ԫ�أ���ԭ����������53��
��4��ﯣ�Zr�������ȵ�1200������ʱ�����ˮ������Ӧ��������ﯣ�ZrO2����������������������ȼ�ձ�ը������������Ӧ�Ļ�ѧ����ʽΪ��Zr+2H2O
| ||
| ||
�ʴ�Ϊ����1�������˶���
��2���٣�
��3���٢ڣ�
��4��Zr+2H2O
| ||
| ||
�����������������¼����������ӵ����ʣ�Ԫ�ص����֪ʶ�ͻ�ѧ����ʽ����д�ȣ����������֪ʶ�Ļ�ѧ���ã���һ����������Ϣ�����⣮

��ϰ��ϵ�д�
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
�����Ŀ
 �ձ��ش������ɸ�����һ�˵�վ1�Ż�����������ը����ش��й����⣺
�ձ��ش������ɸ�����һ�˵�վ1�Ż�����������ը����ش��й����⣺