��Ŀ����
���б����ٺ����нϳ��ĺ����ߣ�������Դʮ�ַḻ��
(1)��ˮɹ�οɻ�ô��Σ���ʵ�����д��ξ����ܽ⡢________��________���Ƶþ��Σ�
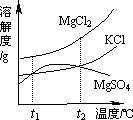
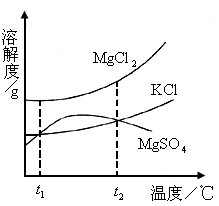
(2)ɹ�κ�õ���±ˮ�к���MgCl2��KCl��MgSO4�����ʣ���ͼ�����ǵ��ܽ������ʾ��ͼ��
����t1��ʱMgCl2��KCl��MgSO4�������ʵ��ܽ�ȷֱ�Ϊa��b��c�������ǵĴ�С��ϵΪ________��
�ڽ�±ˮ���ȵ�t2�����ϣ������ܽ�����ߣ����������ľ�����________��
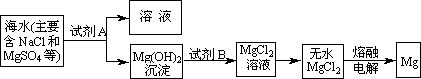
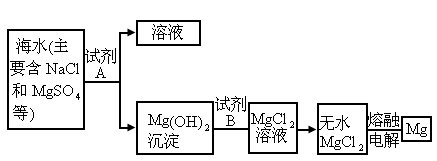
(3)Ŀǰ������60����þ�ǴӺ�ˮ����ȡ�ģ�����Ҫ�������£�

����ȡMg�Ĺ����У��Լ�A����ѡ��________���Լ�Bѡ��________������ˮMgCl2��ȡMg�ķ�Ӧ����Ϊ________��
�ڷ����Mg(OH)2���NaCl��Һ�л�����CaCl2��Na2SO4�����ʣ�Ϊ�˻��NaCl��Һ���ڷ�������Һ�����μ��������BaCl2��Һ��Na2CO3��Һ�����ˣ�������Һ�м����������ᣮʵ���м������BaCl2��Һ��Ϊ�˳�ȥ________���������Na2CO3��Һ��Ŀ����________��
(4)Ŀǰ��ˮ�����ձ���á��༶����������֤������õ���ˮΪ��ˮ�ķ�����________���������Դ����ȼ����������Ҫ�ɷ���ˮ�ϼ��龧��(CH4��nH2O)����ˮ�ϼ��龧����CH4����������Ϊ10������ˮ�ϼ��龧��Ļ�ѧʽΪ________��
������
|
����(1)���ˡ����� ����(2)��a��b��c����MgSO4 ����(3)���������ƻ��������ơ����ᡡ�ֽⷴӦ����Na2SO4����ȥCaCl2������BaCl2 ����(4)ȡ�����μ�AgNO3��Һ����û�а�ɫ������˵������õ���ˮΪ��ˮ��CH4��8H2O |

| |||||||||||||||||||
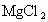 ��KCl��
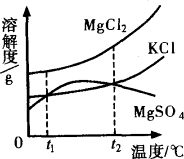
��KCl��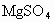 �����ʣ���ͼ�����ǵ��ܽ������ͼ��
�����ʣ���ͼ�����ǵ��ܽ������ͼ��
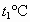 ʱ
ʱ ��KCl��
��KCl��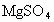 �������ʵ��ܽ�ȷֱ�Ϊa��b��c�������ǵĴ�С��ϵΪ________��
�������ʵ��ܽ�ȷֱ�Ϊa��b��c�������ǵĴ�С��ϵΪ________��
 ���ϣ������ܽ�����ߣ����������ľ�����________��
���ϣ������ܽ�����ߣ����������ľ�����________��
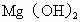 ���NaCl��Һ�л�����
���NaCl��Һ�л�����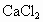 ��
��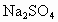 �����ʣ�Ϊ�˻��NaCl��Һ���ڷ�������Һ�����μ��������
�����ʣ�Ϊ�˻��NaCl��Һ���ڷ�������Һ�����μ��������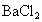 ��Һ��
��Һ��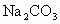 ��Һ�����ˣ�������Һ�м����������ᣮʵ���м������
��Һ�����ˣ�������Һ�м����������ᣮʵ���м������ ��Һ��Ϊ�˳�ȥ________���������
��Һ��Ϊ�˳�ȥ________���������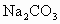 ��Һ��Ŀ����________��
��Һ��Ŀ����________��
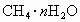 )����ˮ�ϼ��龧����
)����ˮ�ϼ��龧����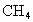 ����������Ϊ10������ˮ�ϼ��龧��Ļ�ѧʽΪ________��
����������Ϊ10������ˮ�ϼ��龧��Ļ�ѧʽΪ________��