��Ŀ����
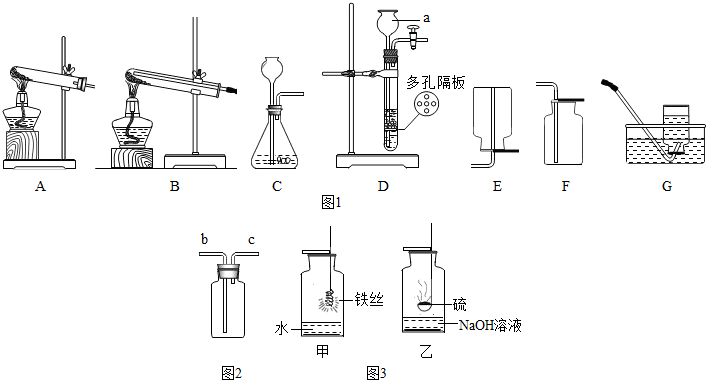
����ʵ��װ��ͼ���ش��������⣺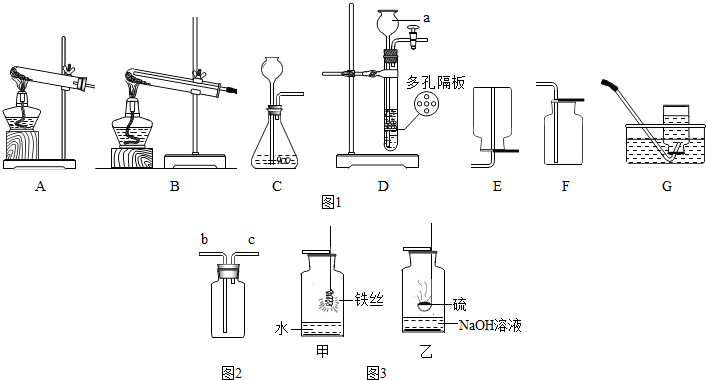
��1��ͼ1�б���a������������______��
��2��ʵ����������غͶ���������ȡ�ϴ�������װ�������______����װ�ñ�ţ�����ѧ����ʽΪ______��
��3��ʵ������п����ϡ������������______����ܡ����ܡ�����װ��D������װ�ã�
��4��������ԭ����ͭ��ʵ���ѡ______װ�ã���װ�ñ�ţ���ʵ��ʱ�����Թ����ѣ���������ԭ�������______��
��5������ʵ������ȡO2��CO2��������ķ�Ӧԭ������ȡ���ռ�������������ɳ�ʵ������ȡ���巴Ӧ�Ĺ�ͬ��______������ţ���
����Ҫ���ȡ��� ��ʹ�ô��������� ��û������μӷ�Ӧ������ ��ԭ��Ϊ�������������ֻ����һ������
��6������ͼ2װ�ý����ſ������ռ�������̼���壬������Ӧ��______����b��c���ڽ��룻
��7��ͼ3��ͬѧ����̽����������ʵ���иĽ���ʵ��װ��ͼ����Ҫ�ش�ס�����������ƿ��Һ�������______��______��
�⣺��1��a�dz���©����
��2������غͶ��������ǹ��壬����װ�ò��ù̹̼���װ�ã���ˮ�����ſ������ռ���������ѡ��������A G����Ӧ�ķ���ʽΪ��2KClO3 2KCl+3 O2����
2KCl+3 O2����
��3��Dװ���ʺϹ�Һ�����͵�������ȡ����������п����ϡ�����ڳ����·�Ӧ�����ܣ����Ҹ�װ�û��ܿ��Ʒ�Ӧ�ķ�����ֹͣ��
��4��������ԭ����ͭ��ʵ�鲽����Ҫ����ͨ��������ֹ��ը�����ⲿ������û�вμӷ�Ӧ������Ҫ�ų�����˲���ѡAװ�ã���ѡ��Bװ�ã��Թܱ�ը��ԭ������У��Թܿ�û��������б�����·�Ӧ�����ɵ�ˮû�м�ʱ�ų������Թ������ˮ��û����������ȵȣ���
��5����ȡ�������Բ��ù���������Һ��ϡ���ᷴӦ����ȡ������̼��ʯ��ʯ��ϡ���ᷴӦ�����ȽϹ�ͬ���ǣ���û������μӷ�Ӧ ��ֻ����һ�����壬��Ҳ����ȡ���������Ĺ�ͬ�㣻
��6����Ϊ������̼���ܶȱȿ�����Ӧ�ôӳ��ܣ�b�����������Ӷ̹��ų���
��7�����е�ˮ���Է�ֹ���ɵ���������������ը��ƿ�ף���Ϊ���������ж������е�Һ��������������Һ������������Һ�����շ�Ӧ���ɵĶ����������壬��ֹ��Ⱦ������
�ʴ�Ϊ����1������©���� ��2��A��G��2KClO3 2KCl+3 O2���� ��3���ܣ� ��4��B���Թܿ�û��������б�����·�Ӧ�����ɵ�ˮû�м�ʱ�ų������Թ������ˮ��û����������ȵȣ�
2KCl+3 O2���� ��3���ܣ� ��4��B���Թܿ�û��������б�����·�Ӧ�����ɵ�ˮû�м�ʱ�ų������Թ������ˮ��û����������ȵȣ�
��5���ۢݣ� ��6��b����7����ֹ�������ۻ����䵽����ƿ�ײ�ʹ����ƿը�ѣ� ����ȼ�ղ����ֹ����������Ⱦ����
��������1���������ջ�ѧ���������������ƺ���;��
��2�����ݷ�Ӧ��״̬�뷴Ӧ����ѡ����װ�ã���������������ܶȺ��ܽ���ѡ���ռ�װ�ã���Ӧ��Ϊ���壻
��3��Dװ���ʺϹ�Һ�����͵�������ȡ��
��4�����������Ŀ�ȼ�Լ�ʵ��ע������ѡ��װ�ã��Թ������������Թֲܾ����Ȳ�������ɵģ�
��5��������Ϣ�Ƚ�������������̼�Ĺ�ͬ�����ѡ��
��6�����ݶ�����̼���ܶȷ�����
��7�����е�ˮ���Է�ֹ���ɵ���������������ը��ƿ�ף����������ж������е�Һ��������������Һ������������Һ�����շ�Ӧ���ɵĶ����������壮
���������⿼�����������ȡ���йص�ע����������֪ʶ��϶࣬��ֻ�и������е�֪ʶ���˽�������Ҫ��ѧ��Ҫ��ʵ������
��2������غͶ��������ǹ��壬����װ�ò��ù̹̼���װ�ã���ˮ�����ſ������ռ���������ѡ��������A G����Ӧ�ķ���ʽΪ��2KClO3
 2KCl+3 O2����
2KCl+3 O2���� ��3��Dװ���ʺϹ�Һ�����͵�������ȡ����������п����ϡ�����ڳ����·�Ӧ�����ܣ����Ҹ�װ�û��ܿ��Ʒ�Ӧ�ķ�����ֹͣ��
��4��������ԭ����ͭ��ʵ�鲽����Ҫ����ͨ��������ֹ��ը�����ⲿ������û�вμӷ�Ӧ������Ҫ�ų�����˲���ѡAװ�ã���ѡ��Bװ�ã��Թܱ�ը��ԭ������У��Թܿ�û��������б�����·�Ӧ�����ɵ�ˮû�м�ʱ�ų������Թ������ˮ��û����������ȵȣ���
��5����ȡ�������Բ��ù���������Һ��ϡ���ᷴӦ����ȡ������̼��ʯ��ʯ��ϡ���ᷴӦ�����ȽϹ�ͬ���ǣ���û������μӷ�Ӧ ��ֻ����һ�����壬��Ҳ����ȡ���������Ĺ�ͬ�㣻
��6����Ϊ������̼���ܶȱȿ�����Ӧ�ôӳ��ܣ�b�����������Ӷ̹��ų���
��7�����е�ˮ���Է�ֹ���ɵ���������������ը��ƿ�ף���Ϊ���������ж������е�Һ��������������Һ������������Һ�����շ�Ӧ���ɵĶ����������壬��ֹ��Ⱦ������
�ʴ�Ϊ����1������©���� ��2��A��G��2KClO3
 2KCl+3 O2���� ��3���ܣ� ��4��B���Թܿ�û��������б�����·�Ӧ�����ɵ�ˮû�м�ʱ�ų������Թ������ˮ��û����������ȵȣ�
2KCl+3 O2���� ��3���ܣ� ��4��B���Թܿ�û��������б�����·�Ӧ�����ɵ�ˮû�м�ʱ�ų������Թ������ˮ��û����������ȵȣ���5���ۢݣ� ��6��b����7����ֹ�������ۻ����䵽����ƿ�ײ�ʹ����ƿը�ѣ� ����ȼ�ղ����ֹ����������Ⱦ����
��������1���������ջ�ѧ���������������ƺ���;��
��2�����ݷ�Ӧ��״̬�뷴Ӧ����ѡ����װ�ã���������������ܶȺ��ܽ���ѡ���ռ�װ�ã���Ӧ��Ϊ���壻
��3��Dװ���ʺϹ�Һ�����͵�������ȡ��
��4�����������Ŀ�ȼ�Լ�ʵ��ע������ѡ��װ�ã��Թ������������Թֲܾ����Ȳ�������ɵģ�
��5��������Ϣ�Ƚ�������������̼�Ĺ�ͬ�����ѡ��
��6�����ݶ�����̼���ܶȷ�����
��7�����е�ˮ���Է�ֹ���ɵ���������������ը��ƿ�ף����������ж������е�Һ��������������Һ������������Һ�����շ�Ӧ���ɵĶ����������壮
���������⿼�����������ȡ���йص�ע����������֪ʶ��϶࣬��ֻ�и������е�֪ʶ���˽�������Ҫ��ѧ��Ҫ��ʵ������

��ϰ��ϵ�д�
 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
�����Ŀ






 ��ijѧ����Ƶ�һ����ϴ������������;��װ�ã�������ˮ�������ռ�����ʱ��ƿ����װ��ˮ�������
��ijѧ����Ƶ�һ����ϴ������������;��װ�ã�������ˮ�������ռ�����ʱ��ƿ����װ��ˮ�������