��Ŀ����
��ͼ������100g��������Ϊ10.6%��NaCl��Һ�Ĺ���ʾ��ͼ��������й����⣺
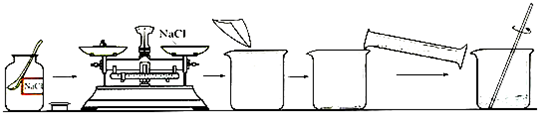
��1����ָ��ͼ���������ԵĴ���˵������������
����٣�
����ڣ�
��2�����㣺NaCl������Ϊ
��3��������
��4���ܽ⣺����Ͳ����ȡ�����ˮ��ˮ���ܶ�Ϊ1g/cm3��������Ͳ�Ĺ���� ����������ѡ�ã�10mL��25mL��50mL��100mL�����ܽ�NaClҪ�õ�����������������
��5����˼������ȡ��NaCl�к���ˮ�֣��������Ƶ���Һ������������
��6������������ͼʾ�������ٶ�����������������������Һ����������

��1����ָ��ͼ���������ԵĴ���˵������������
����٣�
ƿ���������������ˣ�Ӧ�õ���
ƿ���������������ˣ�Ӧ�õ���
������ڣ�
����ʱ������������̣��Ȼ��Ʒ��������̣��ŷ��ˣ�Ӧ�Ȼ��Ʒ�����������������̣�
����ʱ������������̣��Ȼ��Ʒ��������̣��ŷ��ˣ�Ӧ�Ȼ��Ʒ�����������������̣�
����2�����㣺NaCl������Ϊ
10.6
10.6
g��ˮΪ89.4
89.4
g����3��������
��4���ܽ⣺����Ͳ����ȡ�����ˮ��ˮ���ܶ�Ϊ1g/cm3��������Ͳ�Ĺ���� ����������ѡ�ã�10mL��25mL��50mL��100mL�����ܽ�NaClҪ�õ�����������������
�ӿ��ܽ�
�ӿ��ܽ�
����5����˼������ȡ��NaCl�к���ˮ�֣��������Ƶ���Һ������������
ƫС
ƫС
���ƫ����ƫС������Ӱ�족����6������������ͼʾ�������ٶ�����������������������Һ����������
��
��
��ѡ����ڡ�����С�ڡ����ڡ���10.6%����������1������ƿ���ķ��÷�������ƽ����ʱ��������IJ���������
��2������������Ϊ10.6%��֪��100g��Һ�к�����10.6g���ܼ�89.4g���
��4��������Ͳ���ѡ����ȡ��Һ������ӽ���Ͳ�����̶Ȳ�����һ�β����������Һ�������������������Ľ����ܼӿ�ʳ�ε��ܽ������
��5����������ȡ��NaCl�к���ˮ�֣�����Һ�е���������ƫС����Һ��������������������ƫС������
��6�������Ȼ��Ƴ���ʱ��������ʱ���Ȼ��Ƶ�����������������͵������룬������������ʵ������������Ȼ��Ƶ����������Էŷ��˳�ȡ���Ȼ��Ƶ�����С��ʵ�������������������Һ����������ƫС������
��2������������Ϊ10.6%��֪��100g��Һ�к�����10.6g���ܼ�89.4g���
��4��������Ͳ���ѡ����ȡ��Һ������ӽ���Ͳ�����̶Ȳ�����һ�β����������Һ�������������������Ľ����ܼӿ�ʳ�ε��ܽ������
��5����������ȡ��NaCl�к���ˮ�֣�����Һ�е���������ƫС����Һ��������������������ƫС������
��6�������Ȼ��Ƴ���ʱ��������ʱ���Ȼ��Ƶ�����������������͵������룬������������ʵ������������Ȼ��Ƶ����������Էŷ��˳�ȡ���Ȼ��Ƶ�����С��ʵ�������������������Һ����������ƫС������
����⣺��1��ƿ���ķ��÷�������ƽ����ʱ��������IJ������ʴ𰸣�ƿ���������������ˣ�Ӧ�õ��ţ� ����ʱ������������̣��Ȼ��Ʒ��������̣��ŷ��ˣ�Ӧ�Ȼ��Ʒ�����������������̣���
��2����������Ϊ10.6%��֪��100g��Һ�к�����10.6g���ܼ�89.4g���ʴ𰸣�10.6 89.4��
��4����Ͳ���ѡ����ȡ��Һ������ӽ���Ͳ�����̶Ȳ�����һ�β����������Һ�������������������Ľ����ܼӿ�ʳ�ε��ܽ⣬�ʴ𰸣�100mL �ӿ��ܽ⣮
��5������ȡ��NaCl�к���ˮ�֣�����Һ�е���������ƫС����Һ��������������������ƫС���ʴ𰸣�ƫС��
��6���Ȼ��Ƴ���ʱ��������ʱ���Ȼ��Ƶ�����������������͵������룬������������ʵ������������Ȼ��Ƶ����������Էŷ��˳�ȡ���Ȼ��Ƶ�����С��ʵ�������������������Һ����������ƫС���ʴ𰸣�С�ڣ�
��2����������Ϊ10.6%��֪��100g��Һ�к�����10.6g���ܼ�89.4g���ʴ𰸣�10.6 89.4��
��4����Ͳ���ѡ����ȡ��Һ������ӽ���Ͳ�����̶Ȳ�����һ�β����������Һ�������������������Ľ����ܼӿ�ʳ�ε��ܽ⣬�ʴ𰸣�100mL �ӿ��ܽ⣮
��5������ȡ��NaCl�к���ˮ�֣�����Һ�е���������ƫС����Һ��������������������ƫС���ʴ𰸣�ƫС��
��6���Ȼ��Ƴ���ʱ��������ʱ���Ȼ��Ƶ�����������������͵������룬������������ʵ������������Ȼ��Ƶ����������Էŷ��˳�ȡ���Ȼ��Ƶ�����С��ʵ�������������������Һ����������ƫС���ʴ𰸣�С�ڣ�
������������Һ���ֳ����������������ʼ�ˮ�ܽ⣬���Ʋ������-����-�ܽ⣻Һ���ˮϡ�ͣ����Ʋ������-��ȡ-�ܽ⣮

��ϰ��ϵ�д�
�����Ŀ
 ��2012?���ױ����������ͼ���ܽ������ͼ�ش����⣮
��2012?���ױ����������ͼ���ܽ������ͼ�ش����⣮
 �����ͼ���ܽ������ͼ�ش����⣮
�����ͼ���ܽ������ͼ�ش����⣮
