题目内容
某校同学探究附近赤铁矿中Fe2O3的纯度.他们采集了20.0g样品,加入稀盐酸,完全反应后,共用去稀盐酸184.0g,过滤得到滤渣4.0g(假设杂质既不溶于酸,也不溶于水,不考虑实验中的损耗).求:
(1)赤铁矿样品中Fe2O3的质量是多少?其纯度为多少?
(2)反应后所得溶液中溶质的质量分数?
解:(1)赤铁矿样品中Fe2O3的质量=20.0g-4.0g=16.0g;
赤铁矿样品中Fe2O3的质量分数(纯度)=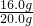 ×100%=80.0%.
×100%=80.0%.
(2)设反应后生成氯化铁的质量为x
Fe2O3+6HCl=2FeCl3+3H2O
160 325
16.0g x
160:325=16.0g:x 解之得 x=32.5g
反应后所得溶液质量=20.0g+184.0g-4.0g=200.0g
所得溶液的溶质质量分数=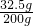 ×100%=16.25%
×100%=16.25%
答:(1)赤铁矿样品中Fe2O3的质量16.0g,其纯度为80.0%;
(2)反应后所得溶液中溶质的质量分数为16.25%.
分析:赤铁矿与盐酸混合,铁矿中氧化铁和盐酸反应,得到氯化铁溶液,铁矿中杂质既不溶酸,也不溶于水,形成滤渣.所以,铁矿中氧化铁的质量可由20.0g-4.0g=16.0g.
点评:反应后溶液质量可根据质量守恒进行计算:反应后溶液质量=赤铁矿质量+稀盐酸质量-滤渣质量
赤铁矿样品中Fe2O3的质量分数(纯度)=
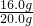 ×100%=80.0%.
×100%=80.0%.(2)设反应后生成氯化铁的质量为x
Fe2O3+6HCl=2FeCl3+3H2O
160 325
16.0g x
160:325=16.0g:x 解之得 x=32.5g
反应后所得溶液质量=20.0g+184.0g-4.0g=200.0g
所得溶液的溶质质量分数=
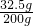 ×100%=16.25%
×100%=16.25%答:(1)赤铁矿样品中Fe2O3的质量16.0g,其纯度为80.0%;
(2)反应后所得溶液中溶质的质量分数为16.25%.
分析:赤铁矿与盐酸混合,铁矿中氧化铁和盐酸反应,得到氯化铁溶液,铁矿中杂质既不溶酸,也不溶于水,形成滤渣.所以,铁矿中氧化铁的质量可由20.0g-4.0g=16.0g.
点评:反应后溶液质量可根据质量守恒进行计算:反应后溶液质量=赤铁矿质量+稀盐酸质量-滤渣质量

练习册系列答案
相关题目