��Ŀ����
���������㣬������ұ�˵���٣��������Ƕ�������Ȱ������˹����㣬�����ܷ⣮Ϊ�˽����ĺ������⣬����ˮ�м���������ƣ���ѧʽΪCaO2��������������ˮ��Ӧ�������������ƺ���������1��д������������ˮ��Ӧ�Ļ�ѧ����ʽ��______��
��2��һλ���㰮�������ⶨ���õĹ���������Ʒ�й������Ƶ�����������������ʵ�飺��ȡ��Ʒ2.0 g�����뵽������ˮ�У�������224 mL�������������ܶ�Ϊ1.43g/L�����Լ���������Ʒ�й������Ƶ�����������
���𰸡���������������ṩ����Ϣ����д���йط�Ӧ�Ļ�ѧ����ʽ����д��ѧ����ʽҪע�⣺�ٻ�ѧʽҪ��ȷ������ѭ�����غ㶨�ɣ���д��Ҫ�����������Ƿ��С�����������
������Ʒ�й������Ƶ���������ʱ�����ݻ�ѧ����ʽ����������Ƶ��������ٳ�����Ʒ������������ã�
����⣺��1��������������ˮ��Ӧ�������ƺ����������Ա����Ϊ��2CaO2+2H2O�T2Ca��OH��2+O2����
��2���⣺224ml��������Ϊ��1.43g/l×0.224l��0.32g
��2.0g��Ʒ�к�CaO2����Ϊa������
2CaO2+2H2O�T2Ca��OH��2+O2��
144 32
a 0.32g
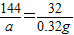
a=1.44g��5�֣�
��Ʒ�й������Ƶ���������Ϊ��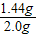 ×100%=72%
×100%=72%
��������Ʒ�й������Ƶ���������Ϊ72%��
����������ע�⿼�黯ѧ����ʽ����д��Ʒ���ȵ����㣬��ɴ��⣬������������ṩ����Ϣ���У���д����ʽҪע����ƽ��
������Ʒ�й������Ƶ���������ʱ�����ݻ�ѧ����ʽ����������Ƶ��������ٳ�����Ʒ������������ã�
����⣺��1��������������ˮ��Ӧ�������ƺ����������Ա����Ϊ��2CaO2+2H2O�T2Ca��OH��2+O2����
��2���⣺224ml��������Ϊ��1.43g/l×0.224l��0.32g
��2.0g��Ʒ�к�CaO2����Ϊa������
2CaO2+2H2O�T2Ca��OH��2+O2��
144 32
a 0.32g
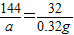
a=1.44g��5�֣�
��Ʒ�й������Ƶ���������Ϊ��
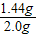 ×100%=72%
×100%=72%��������Ʒ�й������Ƶ���������Ϊ72%��
����������ע�⿼�黯ѧ����ʽ����д��Ʒ���ȵ����㣬��ɴ��⣬������������ṩ����Ϣ���У���д����ʽҪע����ƽ��

��ϰ��ϵ�д�
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
�����Ŀ
