��Ŀ����
��������װ��ͼ��д�йؿո�







 (1)װ��A��C�������٢ڵ�����_______________��______________��
(1)װ��A��C�������٢ڵ�����_______________��______________��
(2)ʵ������ȡ������̼�ķ���װ�ÿ�ѡ������__ _____________������ţ�װ�ã���Aװ����ȡ�����Ļ�ѧ����ʽ_____________��Aװ�������Ķ������Խ���___________(д��Ӧ�Ļ�ѧ����ʽ����ʵ�顣
_____________������ţ�װ�ã���Aװ����ȡ�����Ļ�ѧ����ʽ_____________��Aװ�������Ķ������Խ���___________(д��Ӧ�Ļ�ѧ����ʽ����ʵ�顣
(3)��ȥ���������������ʵ�ʵ�鲽�����ܽ⡢���ˡ����������õ�����װ��ijһװ���⣬����Ҫ�õ����в�����������е�__ ________�������ʵ�顣
________�������ʵ�顣
I��©�����ձ��������� II���ιܡ���Ͳ���Թ� III������ƿ���������ƿ
(4)���ᴿ�õ����Ȼ��ƹ����ˮ����10%���Ȼ�����Һ�����Ƶõ�����Һ��������С��10%�Ŀ���ԭ��______________________________��
(5)��װ��B����25�˴���ʯ��100��ϡ������ȫ��Ӧ�����ʲ���Ӧ����ȡ������̼��װ��B������ʣ�����ʵ�����Ϊ116.2�ˡ��Լ��㣺�ٿ��Ƶ�_______Ħ��������̼���ڲμӷ�Ӧ��HCl�����ʵ����Ƕ��٣�������ݻ�ѧ����ʽ��ʽ���㣩
�۴���ʯ��̼��Ƶİٷֺ���Ϊ_____________��
��1���Թ� ��Һ©��
��2��B��C (д�����ŵ÷֣� �� 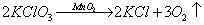

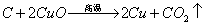 ��
��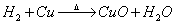 �ȣ����Ͼ͵÷֣�
�ȣ����Ͼ͵÷֣�
(3) I
(4) ��ȡˮʱ���Ӷ�����ʳ�κ�����ŷ��ˡ�������Һ���ձ�����ˮ�ȣ�����������֣� ��2�֣�
(5) 0.2mol ��1�֣�
��μӷ�Ӧ��HCl�����ʵ���Ϊxmol
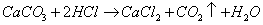 1��
1��
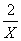
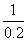
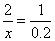
 1��
1��
X=0.4mol 1��
80% ��1�֣�





