题目内容
【题目】已知某化工厂制得的Na2CO3(俗称:纯碱)粗产品中含有杂质氯化钠,为了测定粗产品中纯碱的纯度,进行了如图装置的实验:其中E装置中的饱和NaHCO3溶液是为了除去二氧化碳气体中的氯化氢;发生的反应为NaHCO3+HCl═NaCl+H2O+CO2↑.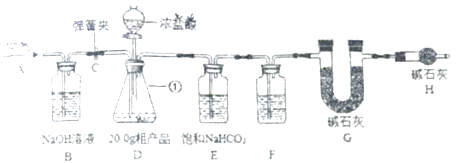
①连接好装置,检查气密性;将20g粗产品放入锥形瓶中,加适量蒸馏水溶解,得到试样溶液;
②打开弹簧夹C,在A处缓缓通入一段时间空气;
③称量 G的质量;
④关闭弹簧夹C,慢慢滴加浓盐酸直至D中无气泡冒出;
⑤打开弹簧夹C,再次缓缓通入一段时间空气;
⑥再次称量G的质量,得前后两次质量差为4.8g.
请回答下列问题
(1)写出仪器①的名称;F中的试剂应为 .
(2)B装置的作用是 , B装置中发生反应的化学方程式为 .
(3)在步骤②中,A处缓缓通入一段时间空气的目的是 .
(4)若没有H装置,测定的Na2CO3的质量分数会(填“偏大”、“偏小”、“不变”).
(5)该兴趣小组指导老师认为该方案有些不合理,指导同学们更换了共中的一种试剂并去掉了一个装置后实验得到了改善,并测得20.0g粗产品只能产生4.4gCO2 . 你认为更换后的试剂是 . 原实验中实验值4.8g比正确值4.4g偏大的原因是(假设操作均正确) . 数据计算:根据正确值4.4g求得了样品中Na2CO3的质量分数 .
【答案】
(1)锥形瓶,浓硫酸
(2)除去空气中的二氧化碳,2NaOH+CO2═Na2CO3+H2O
(3)排出装置中空气
(4)偏大
(5)稀硫酸,浓盐酸有挥发性,产生二氧化碳中含HCl气体与碳酸氢钠溶液反应又生成了二氧化碳,53%
【解析】(1)仪器①的名称锥形瓶;因为浓硫酸能吸收水蒸气,故F中的试剂应为浓硫酸;
(2)氢氧化钠溶液能吸收空气中的二氧化碳,故B装置中发生反应的化学方程式为2NaOH+CO2═Na2CO3+H2O;
(3)向A装置中通入气体可以将装置中的气体排净,防止干扰实验现象及结论;
(4)H装置能防止空气中的二氧化碳和水蒸气进入G装置,若没有H装置,则测定的二氧化碳值偏高,计算出的质量分数偏大;(5)装置D中浓盐酸挥发出的氯化氢与装置E中NaHCO3反应产生二氧化碳,使二氧化碳的值偏大,为了使实验结果更准确防止干扰,可以把浓盐酸换成稀硫酸,
设碳酸钠的质量为x.
Na2CO3+ | 2HCl= | 2NaCl+H2O+ | CO2↑ |
106 | 44 | ||
x | 4.4g |
则 ![]() ,解得:x=10.6g.
,解得:x=10.6g.
碳酸钠的质量分数为: ![]() ×100%=53%.
×100%=53%.
故答案为:(1)锥形瓶; 浓硫酸;(2)除去空气中的二氧化碳;2NaOH+CO2═Na2CO3+H2O;(3)排出装置中空气;(4)偏大;(5)稀硫酸;浓盐酸有挥发性,产生二氧化碳中含HCl气体与碳酸氢钠溶液反应又生成了二氧化碳;53%.
(1)根据常用仪器的名称解答即可;
(2)氢氧化钠溶液能吸收空气中的二氧化碳;
(3)浓盐酸能够挥发出氯化氢;
(4)H装置能防止空气中的二氧化碳和水蒸气进入G装置,若没有H装置,则测定的二氧化碳值偏高;
(5)写出碳酸钠和稀盐酸反应的化学反应方程式,然后结合二氧化碳的质量即可求出碳酸钠的质量,最后用碳酸钠的质量除以粗产品的质量再乘以100%即可求出样品中Na2CO3的质量分数.

 同步练习河南大学出版社系列答案
同步练习河南大学出版社系列答案 同步练习西南师范大学出版社系列答案
同步练习西南师范大学出版社系列答案 补充习题江苏系列答案
补充习题江苏系列答案 学练快车道口算心算速算天天练系列答案
学练快车道口算心算速算天天练系列答案