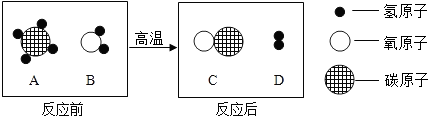
��Ŀ����
��ʵġ�̼�������˵����������һ���߽���̼�������磮
��1�������±��ṩ����Ϣ����д�йغ�̼���ʶ�Ӧ���������ʣ�
��2��Һ̬������̼������������˾ȵ��������ҷ����Ļ��֣�����˵����ȷ���� ������ĸ��ţ���
A��Һ̬������̼��������Ⱦ��������
B��������̼�ɸ�����ȼ������棬��������
C��Һ̬������̼����ʱ���ȣ������˿�ȼ����Ż��
��3������Ķ�����̼�Ӿ��ˡ�����ЧӦ����д��һ�����ٶ�����̼�ŷŵĽ��� ��
��4��������̼��һ�ֱ������Դ���̶������ö�����̼��һ���ɹ������ǣ��ڸ��¸�ѹ�£�CO2��NH3���Ժϳ�����[CO��NH2��2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5����������Ŵ����ġ���ȼ����������Ϊδ��������Դ������Ҫ�ɷ��Ǽ���ˮ���������ȫȼ�յĻ�ѧ����ʽΪ ��
��6��ʯ��ʯ��;�dz��㷺�������������ջ�������糧úȼ��ʱ�����Ķ�������������뽫��Ӧ�Ļ�ѧ����ʽ����������2CaCO3+2SO2+O2
2CaSO3+2 ��
��1�������±��ṩ����Ϣ����д�йغ�̼���ʶ�Ӧ���������ʣ�
| ������; | ���ʯ�и�� | ʯī���缫 | ����̿��ˮ |
| ��Ӧ�������� | �� |
�� |
�� |
A��Һ̬������̼��������Ⱦ��������
B��������̼�ɸ�����ȼ������棬��������
C��Һ̬������̼����ʱ���ȣ������˿�ȼ����Ż��
��3������Ķ�����̼�Ӿ��ˡ�����ЧӦ����д��һ�����ٶ�����̼�ŷŵĽ���
��4��������̼��һ�ֱ������Դ���̶������ö�����̼��һ���ɹ������ǣ��ڸ��¸�ѹ�£�CO2��NH3���Ժϳ�����[CO��NH2��2]��ͬʱ����ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ
��5����������Ŵ����ġ���ȼ����������Ϊδ��������Դ������Ҫ�ɷ��Ǽ���ˮ���������ȫȼ�յĻ�ѧ����ʽΪ
��6��ʯ��ʯ��;�dz��㷺�������������ջ�������糧úȼ��ʱ�����Ķ�������������뽫��Ӧ�Ļ�ѧ����ʽ����������2CaCO3+2SO2+O2
| ||
��������1�����ʯ����Ȼ���ڵ�Ӳ���������ʣ�ʯī���������ĵ����ԣ�����̿���������ԣ�
��2�����ݶ�����̼����ԭ�����н��
��3�����ݼ��ٶ�����̼�ŷŵĴ�ʩ�У����С���Լ��ֽ�Ƚ��н��
��4������CO2��NH3��Ӧ�������غ�ˮ�ķ�Ӧԭ����д����ʽ��
��5�����ݼ���ȼ�յķ�Ӧԭ����д����ʽ��
��6���ݷ�Ӧǰ��ԭ�ӵ��������Ŀ�����ж����ʵĻ�ѧʽ��
��2�����ݶ�����̼����ԭ�����н��
��3�����ݼ��ٶ�����̼�ŷŵĴ�ʩ�У����С���Լ��ֽ�Ƚ��н��
��4������CO2��NH3��Ӧ�������غ�ˮ�ķ�Ӧԭ����д����ʽ��
��5�����ݼ���ȼ�յķ�Ӧԭ����д����ʽ��
��6���ݷ�Ӧǰ��ԭ�ӵ��������Ŀ�����ж����ʵĻ�ѧʽ��
����⣺��1�����ʯ����Ȼ���ڵ�Ӳ���������ʣ��������и����ʯī���е����ԣ��������缫������̿���������ԣ�����������ˮ�е�ɫ�ء���ζ�Ƚ��о�ˮ��
��2��A��Һ̬������̼��������Ⱦ�������ϣ���A��ȷ��
B��������̼�ܶȱȿ����ɸ�����ȼ������棬�����������Ӷ����������ã���B��ȷ��
C��Һ̬������̼����ʱ���ȣ����ǿ�ȼ����Ż���Dz���ģ����ܽ��ͣ���C����
��3�����ٶ�����̼�ŷŵĴ�ʩ���Բ�������˫��ʵ��ֽ�š���Լ��ֽ�ȣ�
��4��CO2��NH3��Ӧ�������غ�ˮ���÷�Ӧ�Ļ�ѧ����ʽΪCO2+2NH3
CO��NH2��2+H2O��
��5�������ڵ�ȼ�����������ɶ�����̼��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪCH4+2O2
CO2+2H2O��
��6���۲췴Ӧ����ʽ��2CaCO3+2SO2+O2
2CaSO3+2������������֪��Ӧǰ�и�ԭ��2����̼ԭ��2������ԭ��12������ԭ��2�������Ӧ���ʵ�ÿ��������һ��̼ԭ�Ӻ�������ԭ�ӣ�����Ϊ������̼��
�ʴ�Ϊ������1����Ӳ�ȴ� �ڵ����� ��������
��2��AB
��3���Բ���������Լ��ֽ�ȣ��������ɣ�
��4��CO2+2NH3
CO��NH2��2+H2O ����2�֣�����ѧʽ����0�֣���ƽ���������ۣ�1�֣�����ͬ��
��5��CH4+2O2
CO2+2H2O
��6��CO2��
��2��A��Һ̬������̼��������Ⱦ�������ϣ���A��ȷ��
B��������̼�ܶȱȿ����ɸ�����ȼ������棬�����������Ӷ����������ã���B��ȷ��
C��Һ̬������̼����ʱ���ȣ����ǿ�ȼ����Ż���Dz���ģ����ܽ��ͣ���C����
��3�����ٶ�����̼�ŷŵĴ�ʩ���Բ�������˫��ʵ��ֽ�š���Լ��ֽ�ȣ�
��4��CO2��NH3��Ӧ�������غ�ˮ���÷�Ӧ�Ļ�ѧ����ʽΪCO2+2NH3
| ||
��5�������ڵ�ȼ�����������ɶ�����̼��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪCH4+2O2
| ||
��6���۲췴Ӧ����ʽ��2CaCO3+2SO2+O2
| ||
�ʴ�Ϊ������1����Ӳ�ȴ� �ڵ����� ��������
��2��AB
��3���Բ���������Լ��ֽ�ȣ��������ɣ�
��4��CO2+2NH3
| ||
��5��CH4+2O2
| ||
��6��CO2��
������������Ҫ�����˷���ʽ����д�������غ㶨�ɵ�Ӧ�á�����ЧӦ��֪ʶ���˽���ֻ�������IJ����Լ���Ӧ�ķ��δ�ʩ����ȷ����ʽ����д�������ǽ���������Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
 ��2013?���ݣ���ʵġ�̼�������˵����������һ���߽���̼�������磮
��2013?���ݣ���ʵġ�̼�������˵����������һ���߽���̼�������磮 ��ʵġ�̼�������˵����������һ���߽���̼�������磮
��ʵġ�̼�������˵����������һ���߽���̼�������磮 ��ʵġ�̼�������˵����������һ���߽���̼�������磮
��ʵġ�̼�������˵����������һ���߽���̼�������磮