��Ŀ����
 ijͬѧ������������������Ϊ3%�Ĺ���������Һ��Ϊ����Һ������һƿ��ǩ������Ĺ���������Һ��Ϊ�ⶨƿ����Һ�����ʵ�����������ȡ����Һ 35.0g���ձ��У�����һ�����Ķ������̣���ȫ��Ӧ�Ƶ��ձ���ʣ�����ʵ���������35.8g��������ʣ�����ʹ��ˡ�ϴ�ӡ�����������4.0g�������Ҫ��ش��������⣺
ijͬѧ������������������Ϊ3%�Ĺ���������Һ��Ϊ����Һ������һƿ��ǩ������Ĺ���������Һ��Ϊ�ⶨƿ����Һ�����ʵ�����������ȡ����Һ 35.0g���ձ��У�����һ�����Ķ������̣���ȫ��Ӧ�Ƶ��ձ���ʣ�����ʵ���������35.8g��������ʣ�����ʹ��ˡ�ϴ�ӡ�����������4.0g�������Ҫ��ش��������⣺��1������������������
3.2g
3.2g
����2������ƿ�ڹ���������Һ�����ʵ�����������
��������1�����������غ㶨�ɿ�֪��Һ���ٵ�������������������������
��2����������������ȡ����������Һ���������ݹ�������ֽ�Ļ�ѧ����ʽ���Լ����ƿ�ڹ���������Һ�����ʵ�����������
��2����������������ȡ����������Һ���������ݹ�������ֽ�Ļ�ѧ����ʽ���Լ����ƿ�ڹ���������Һ�����ʵ�����������
����⣺��1���ɷ�Ӧ�������֪����ʣ�����ʹ��ˡ�ϴ�ӡ�����������4.0g��Ϊ�������̵����������������غ㶨�ɿ�֪��������������Ϊ��35g+4g-35.8g=3.2g
��2����ƿ�ڹ���������Һ�����ʵ���������Ϊx��
2H2O2
2H2O+O2��
68 32
35g��x 3.2g
=
x=19.4%
�ʴ�Ϊ����1��3.2g��
��2��ƿ�ڹ���������Һ�����ʵ���������Ϊ19.4%��
��2����ƿ�ڹ���������Һ�����ʵ���������Ϊx��
2H2O2
| ||
68 32
35g��x 3.2g
| 68 |
| 35g��x |
| 32 |
| 3.2g |
x=19.4%
�ʴ�Ϊ����1��3.2g��
��2��ƿ�ڹ���������Һ�����ʵ���������Ϊ19.4%��
������������Ҫ����ѧ�����������غ㶨�ɡ���ѧ����ʽ����������������ʽ���м����������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
 Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ
 ijͬѧ������������������Ϊ3%�Ĺ���������Һ��Ϊ����Һ������һƿ��ǩ������Ĺ���������Һ��Ϊ�ⶨƿ����Һ�����ʵ�����������ȡ����Һ34g���ձ��У�����һ�����Ķ������̣���ȫ��Ӧ�Ƶ��ձ���ʣ�����ʵ���������33.8g��������ʣ�����ʹ��ˡ�ϴ�ӡ�����������3g�����㣺
ijͬѧ������������������Ϊ3%�Ĺ���������Һ��Ϊ����Һ������һƿ��ǩ������Ĺ���������Һ��Ϊ�ⶨƿ����Һ�����ʵ�����������ȡ����Һ34g���ձ��У�����һ�����Ķ������̣���ȫ��Ӧ�Ƶ��ձ���ʣ�����ʵ���������33.8g��������ʣ�����ʹ��ˡ�ϴ�ӡ�����������3g�����㣺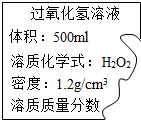 ijͬѧ������������������Ϊ3%�Ĺ���������Һ��Ϊ����Һ������һƿ��ǩ������Ĺ���������Һ��Ϊ�ⶨƿ����Һ�����ʵ�����������ȡ����Һ34g���ձ��У�����һ�����Ķ������̣���ȫ��Ӧ�Ƶ��ձ���ʣ�����ʵ���������33.8g��������ʣ�����ʹ��ˡ�ϴ�ӡ�����������3g�������Ҫ��ش��������⣺
ijͬѧ������������������Ϊ3%�Ĺ���������Һ��Ϊ����Һ������һƿ��ǩ������Ĺ���������Һ��Ϊ�ⶨƿ����Һ�����ʵ�����������ȡ����Һ34g���ձ��У�����һ�����Ķ������̣���ȫ��Ӧ�Ƶ��ձ���ʣ�����ʵ���������33.8g��������ʣ�����ʹ��ˡ�ϴ�ӡ�����������3g�������Ҫ��ش��������⣺ ijͬѧ������������������Ϊ3%�Ĺ���������Һ��Ϊ����Һ������һƿ��ǩ������Ĺ���������Һ��Ϊ�ⶨƿ����Һ�����ʵ�����������ȡ����Һ34g���ձ��У�����һ�����Ķ������̣���ȫ��Ӧ�Ƶ��ձ���ʣ�����ʵ���������33.8g��������ʣ�����ʹ��ˡ�ϴ�ӡ�����������3g�������Ҫ��ش��������⣺
ijͬѧ������������������Ϊ3%�Ĺ���������Һ��Ϊ����Һ������һƿ��ǩ������Ĺ���������Һ��Ϊ�ⶨƿ����Һ�����ʵ�����������ȡ����Һ34g���ձ��У�����һ�����Ķ������̣���ȫ��Ӧ�Ƶ��ձ���ʣ�����ʵ���������33.8g��������ʣ�����ʹ��ˡ�ϴ�ӡ�����������3g�������Ҫ��ش��������⣺ ijͬѧ������������������Ϊ3%�Ĺ���������Һ��Ϊ����Һ������һƿ��ǩ������Ĺ���������Һ��Ϊ�ⶨƿ����Һ�����ʵ�����������ȡ����Һ34g���ձ��У�����һ�����Ķ������̣���ȫ��Ӧ�Ƶ��ձ���ʣ�����ʵ���������33.8g��������ʣ�����ʹ��ˡ�ϴ�ӡ�����������3g�����㣺
ijͬѧ������������������Ϊ3%�Ĺ���������Һ��Ϊ����Һ������һƿ��ǩ������Ĺ���������Һ��Ϊ�ⶨƿ����Һ�����ʵ�����������ȡ����Һ34g���ձ��У�����һ�����Ķ������̣���ȫ��Ӧ�Ƶ��ձ���ʣ�����ʵ���������33.8g��������ʣ�����ʹ��ˡ�ϴ�ӡ�����������3g�����㣺