��Ŀ����
 ��������A��B��С�⣬������ѡһ���𣬲������ⶼ�⣮�����ⶼ�⣬����AС��Ʒ֣�
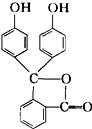
��������A��B��С�⣬������ѡһ���𣬲������ⶼ�⣮�����ⶼ�⣬����AС��Ʒ֣�A����̪��һ�����ָʾ�������Ľṹ��ͼ��ʾ�����̪�Ļ�ѧʽΪ
B�������Ʋ��ǻ�ѧ�о���һ����Ҫ�ֶΣ�A��B��C����ѧ��ѧ�г�����һ�ֻ��������C��һ���Σ���֪��A+nB=C+H2O������ʽ����ƽ����nΪ��ѧ����������ش��������⣺
��1���û�ѧ����ʽ�Ļ�ѧ������֮��Ϊ
��2����n=1ʱ����B�����ᣬC����Է�������Ϊ120���Ʋ�C�Ļ�ѧʽΪ
��3����n=2ʱ����A��һ�ֳ����Ľ����������ٳ�һ�����������Ļ�ѧ����ʽ
������A�����ݷ�̪�Ľṹʽ���ж����Ԫ�ؼ�Ԫ��ԭ�ӵĸ�������ȷ����ѧʽ��������ṹ���ж����ʵ����Ȼ�����û�ѧʽ�����ԭ������������Ԫ�ص�����������
B�������Ѿ���ƽ�Ļ�ѧ��Ӧ����ʽΪA+nB=C+H2O�������㻯ѧ������֮�ͣ������غ��C����Է�������Ϊ120���ƶ�C�Ļ�ѧʽ������n=2ʱ��A��һ�ֳ����Ľ����������֪����Ϊ+2�ۡ���ΪһԪ������дһ�����������Ļ�ѧ����ʽ��
B�������Ѿ���ƽ�Ļ�ѧ��Ӧ����ʽΪA+nB=C+H2O�������㻯ѧ������֮�ͣ������غ��C����Է�������Ϊ120���ƶ�C�Ļ�ѧʽ������n=2ʱ��A��һ�ֳ����Ľ����������֪����Ϊ+2�ۡ���ΪһԪ������дһ�����������Ļ�ѧ����ʽ��
����⣺A���ɽṹʽ��֪������������C��H��O����Ԫ����ɣ���1����������20��Cԭ�ӡ�14��Hԭ�ӡ�4��Oԭ�ӣ�
��ѧʽΪC20H14O4���������Ǻ�̼�Ļ����������������ϴ����л����������ƣ��������л��
������Ԫ�ص���������Ϊ
��100%=20.1%���ʴ�Ϊ��C20H14O4���л��20.1%��
B����1���ɻ�ѧ����ʽΪA+nB=C+H2O��A��C��H2O�Ļ�ѧ����������1��B�Ļ�ѧ������Ϊn����ѧ������֮��Ϊ
n+3���ʴ�Ϊ��n+3��
��2��n=1ʱ����B�����ᣬ��������غ㣬��C�к�����������ɻ�ѧ��������C�Ļ�ѧʽΪMSO4���ٸ���C�����
��������Ϊ120����M�����ԭ������Ϊ120-96=24�������Ϊþ��C�Ļ�ѧʽΪMgSO4����Ϊ��MgSO4��
��3����n=2ʱ������ӦΪһԪ�ᣬ��ѡ�����ᣬC�Ļ�ѧ������Ϊ1�������Ϊ+2�۽��������ѡ������þ�����ᷴӦ��
�ʴ�Ϊ��MgO+2HCl�TMgCl2+H2O��
��ѧʽΪC20H14O4���������Ǻ�̼�Ļ����������������ϴ����л����������ƣ��������л��
������Ԫ�ص���������Ϊ
| 16��4 |
| 12��20+1��14+16��4 |
B����1���ɻ�ѧ����ʽΪA+nB=C+H2O��A��C��H2O�Ļ�ѧ����������1��B�Ļ�ѧ������Ϊn����ѧ������֮��Ϊ
n+3���ʴ�Ϊ��n+3��
��2��n=1ʱ����B�����ᣬ��������غ㣬��C�к�����������ɻ�ѧ��������C�Ļ�ѧʽΪMSO4���ٸ���C�����
��������Ϊ120����M�����ԭ������Ϊ120-96=24�������Ϊþ��C�Ļ�ѧʽΪMgSO4����Ϊ��MgSO4��
��3����n=2ʱ������ӦΪһԪ�ᣬ��ѡ�����ᣬC�Ļ�ѧ������Ϊ1�������Ϊ+2�۽��������ѡ������þ�����ᷴӦ��
�ʴ�Ϊ��MgO+2HCl�TMgCl2+H2O��
���������⿼��ѧ������Ϣϰ��ķ����ͽ��ѧ��Ӧѧ���ȡ��Ϣ�еĹؼ���Ϣ�������ѧ֪ʶ�����ע���غ㷨�ڽ���е�Ӧ�ã�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 33����������a��b��С�⣬��ѡһ���𣬲������ⶼ�⣮�����ⶼ�⣬����aС��Ƿ֣�
33����������a��b��С�⣬��ѡһ���𣬲������ⶼ�⣮�����ⶼ�⣬����aС��Ƿ֣�
 ��������a��b��С�⣬������ѡһ���𣬲������ⶼ�⣮�����ⶼ�⣬����aС��Ʒ֣�
��������a��b��С�⣬������ѡһ���𣬲������ⶼ�⣮�����ⶼ�⣬����aС��Ʒ֣�