��Ŀ����
д�����·�Ӧ�Ļ�ѧ����ʽ��1������ƽ�����ʢ��ǿ��ԭ��(N2H4)��ǿ������˫��ˮ(H2O2)�������ǻ��ʱ������������������ˮ����_______�����ų������ȡ������ҹ��Ļ������ߣ������Ӧ��һ���ܴ��ŵ���_______��
��2���г����۵ĸ�����Ƭ�к������ġ�������ϸ�����ۣ�ʳ�ú���θҺ����������ᣩ��ת��Ϊ��������������Σ��Է�ȱ����ƶѪ_______��
��3��������������ը_______��
��4���ҹ��Ŵ��С���ɰ��֮��ˮ����֮˵����ָ��ɰ���������ȷ����ֽⷴӦ_______��
������
| ������1��N2H4+2H2O2=N2+4H2O û��Ⱦ
��2��Fe+2HCl=FeCl2+H2 ��3��2H2+O2 ��4��HgS
|
��ʾ��

 ��У����ϵ�д�
��У����ϵ�д�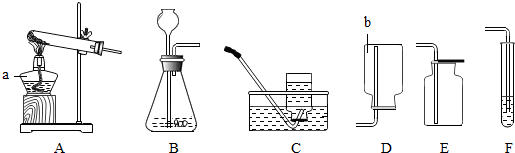
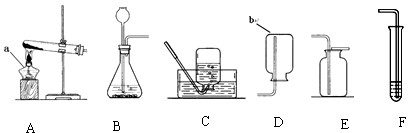
��1����д���б�ŵ��������ƣ�a
��2����ʵ�����ͨ�������з�������������˫��ˮ�Ͷ������̻�ϣ�������غͶ������̻�ϼ��ȣ��ۼ��ȸ�����أ������������֡����ܡ���������̼��������Ʊ�������
��3������A��Cװ����ȡ����ʱ������ˮ���е�ˮ����Ϻ�ɫ��Ϊ�������������Ӧ�Ը�ʵ��װ��������һ��Ķ���
��4��Ϊ����CO2���壬�ɰ�����ͨ��Fװ���У���F�е��Լ���
��5���Ķ��������ϲ��ش�
| ���� | ��ȡ�����ҩƷ | ��Ӧ���� | ������������� |
| ���� | �������̹��塢 Ũ���� |
��Ҫ���� | �ܶȱȿ����� ������ˮ |
ͼ10��ʵ���ҳ��õ�ʵ��������װ�ã�������ѧ֪ʶ�ش��������⣺

��1����д���б�ŵ��������ƣ�a b
��2����ʵ�����ͨ�������з�������������˫��ˮ�Ͷ������̻�ϣ�������غͶ������̻�ϼ��ȣ��ۼ��ȸ�����أ������������֡����ܡ���������̼��������Ʊ�������__ __������ţ���д���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��3������A��Cװ����ȡ����ʱ������ˮ���е�ˮ����Ϻ�ɫ��Ϊ�������������Ӧ�Ը�ʵ��װ��������һ��Ķ��� ��
��4��Ϊ����CO2���壬�ɰ�����ͨ��Fװ���У���F�е��Լ���____ ______�� F�з�Ӧ��������_______ _______________��
��5���Ķ��������ϲ��ش�
| ���� | ��ȡ�����ҩƷ | ��Ӧ���� | ������������� |
| ���� | �������̹��塢 Ũ���� | ��Ҫ���� | �ܶȱȿ����� ������ˮ |
��ȡ����____________������ԡ������ԡ�����ͬ������Bװ�ã��ռ�����_____ ______����Eװ�á�
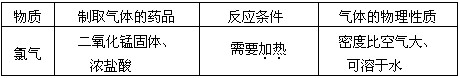
��ͬѧΪ��̽����������ͭ���ֽ����Ļ�ԣ�����ͭ˿����˿����˿��ϡ���������Լ������������ֻ��һ֧�Թܣ�ȡһ�������̽��������
��1�����������ͬѧ�����±��е�̽������
| ʵ�鲽�� | �۲쵽������ |
| �����Թ���ȡ�������ᣬ������˿��������� |
|
| ���ڢ�������Һ�У�����________��������� | ���������� |
| ���ڢ�������Һ�У�����________��������� |
|
���ۣ��������Al>Fe> Cu
д�����з�Ӧ�Ļ�ѧ����ʽ��_____________________________________________��
����˿������ҺǰӦ���еIJ�����_________________________________________��
��2����ͬѧ������ͬѧ�ķ�������ΪֻҪ�ٲ���һ��ʵ�飬���ܵó����Al>Fe>H >Cu�Ľ��ۣ���ͬѧҪ�����ʵ���� ��
��3����ͬѧ��ΪҪ�õ����Al>Fe>H >Cu�Ľ��ۣ����ز���ʵ�飬ֻ�轫��ͬѧ�����в��������˳��������ɣ�������Ľ�������˳��Ϊ ��