��Ŀ����
С��ͬѧΪ�˲ⶨ����С�մ��θҩ��С�մ�ĺ���������θҩ�������ɷֲ�����ˮ��Ҳ�����ᷴӦ��ȡ20g��θҩ���ձ��У������м���100gϡ���ᣬǡ����ȫ��Ӧ���ձ���ʣ�����ʵ�����Ϊ111.2g�������Ҫ��ش����⣺��1��������Ӧ�Ļ�ѧ����ʽ______
��2��������֪�����г�ϡ����������������x���ı���ʽ______
��3����θҩ��С�մ�ĺ���Ϊ______
��4����С����ȡһ�ֺ�С�մ��θҩ�������ɷֲ�����ˮҲ�����ᷴӦ�������ƵIJⶨ��ͬ��ȡ20gҩƷ��100g��һ��δ֪Ũ�ȵ����ᣬǡ����ȫ��Ӧ�����ձ���ʣ����ҺΪ106g�������������������������Ϊ______ ��ȡ������
���𰸡���������1������С�մ�����ʣ�д��С�մ������ᷢ����Ӧ�Ļ�ѧ����ʽ��
��2�����������غ㶨�ɣ������ǡ����ȫ��Ӧ���ų�������̼�����������ö�����̼�������ڷ�Ӧ�е�������ϵ���г�ϡ����������������x���ı���ʽ��
��3����θҩ��С�մ�ĺ���= ×100%������С�մ�������ɸ��ݷ�Ӧ�Ļ�ѧ����ʽ���ɶ�����̼�������������
×100%������С�մ�������ɸ��ݷ�Ӧ�Ļ�ѧ����ʽ���ɶ�����̼�������������
��4�����ݷ�Ӧ�Ļ�ѧ����ʽ���ɼ���̼�����Ƶ�������ų�������̼��������ϵ���ж���ȫ��Ӧʱ��Һ�����IJ���������Һ������������ķ�Ӧ��ϵ�����òⶨʵ������Һǰ�����������������ϡ���������������
����⣺��1��̼�����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪNaHCO3+HCl�TNaCl+H2O+CO2����
��2�����������غ㶨�ɣ���Ӧ�ų�������̼������=20g+100g-111.2g=8.8g
NaHCO3+HCl�TNaCl+H2O+CO2��
84 36.5 44
x 8.8g
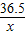 =
=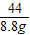
��3����С�մ������Ϊy
NaHCO3+HCl�TNaCl+H2O+CO2��
84 44
y 8.8g
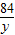 =
=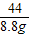 y=16.8g
y=16.8g
��θҩ��С�մ�ĺ���=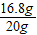 ×100%=84%
×100%=84%
��4�����������غ㶨�ɣ���Ӧ�ų�������̼����Һ��������=106g-100g=6g
�����������������������Ϊz
NaHCO3+HCl�TNaCl+H2O+CO2�� ��Һ����
84 36.5 44 84-44=40
100g×z 6g
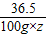 =
=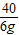 z=5%
z=5%
�ʴ�Ϊ��
��1��NaHCO3+HCl�TNaCl+H2O+CO2����
��2��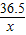 =
=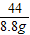 ��
��
��3��84%��
��4��5%��
������������Һ�IJ�����������⣺���ݷ�Ӧ�Ļ�ѧ����ʽ�����÷�Ӧǰ��������Һ����������㷴Ӧ�����������������������������ʹ������̼������࣮
��2�����������غ㶨�ɣ������ǡ����ȫ��Ӧ���ų�������̼�����������ö�����̼�������ڷ�Ӧ�е�������ϵ���г�ϡ����������������x���ı���ʽ��
��3����θҩ��С�մ�ĺ���=
 ×100%������С�մ�������ɸ��ݷ�Ӧ�Ļ�ѧ����ʽ���ɶ�����̼�������������
×100%������С�մ�������ɸ��ݷ�Ӧ�Ļ�ѧ����ʽ���ɶ�����̼���������������4�����ݷ�Ӧ�Ļ�ѧ����ʽ���ɼ���̼�����Ƶ�������ų�������̼��������ϵ���ж���ȫ��Ӧʱ��Һ�����IJ���������Һ������������ķ�Ӧ��ϵ�����òⶨʵ������Һǰ�����������������ϡ���������������
����⣺��1��̼�����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪNaHCO3+HCl�TNaCl+H2O+CO2����
��2�����������غ㶨�ɣ���Ӧ�ų�������̼������=20g+100g-111.2g=8.8g
NaHCO3+HCl�TNaCl+H2O+CO2��
84 36.5 44
x 8.8g
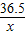 =
=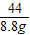
��3����С�մ������Ϊy
NaHCO3+HCl�TNaCl+H2O+CO2��
84 44
y 8.8g
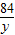 =
=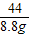 y=16.8g
y=16.8g��θҩ��С�մ�ĺ���=
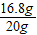 ×100%=84%
×100%=84%��4�����������غ㶨�ɣ���Ӧ�ų�������̼����Һ��������=106g-100g=6g
�����������������������Ϊz
NaHCO3+HCl�TNaCl+H2O+CO2�� ��Һ����
84 36.5 44 84-44=40
100g×z 6g
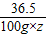 =
=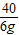 z=5%
z=5%�ʴ�Ϊ��
��1��NaHCO3+HCl�TNaCl+H2O+CO2����
��2��
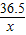 =
=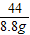 ��
����3��84%��
��4��5%��
������������Һ�IJ�����������⣺���ݷ�Ӧ�Ļ�ѧ����ʽ�����÷�Ӧǰ��������Һ����������㷴Ӧ�����������������������������ʹ������̼������࣮

��ϰ��ϵ�д�
�����Ŀ