��Ŀ����
 ��2013?��������������ʯ����������ɵģ�
��2013?��������������ʯ����������ɵģ���1��ʯ������ˮ�У�����ˮ���ϣ�˵��ʯ�����ܶȱ�ˮ���ܶ�
С
С
�����С��������2����ȼ��������ȼ�չ����У����������仯����
�����ۻ�
�����ۻ�
����3��ȡһ�����סһ��Ѹ��ƽ���������Լ1���ȡ�����ɹ۲쵽���˱�ڣ��м������Ա仯��˵�������
����
����
������桱�����桱�����ġ��������¶���ߣ���4������һ������ʹ����Ϩ��ԭ����
C
C
������ţ���A������ʯ�����Ż�� B�������˿��� C���¶Ƚ������Ż�����£�
��������1������ʵ������Ϊʯ������ˮ�����ж���������ʣ��ɽ��˵�������⣻
��2�����������仯�������ͻ�ѧ�仯���ص���н��
��3�����ݻ���ױ�̿���ij̶�����������Ļ����¶ȣ�
��4��ȼ�յ���Ҫ��֮һ����ȼ������Ҫ���¶ȣ�������Ĺ����л���������ϵĴ���������ʹ���¶ȼ��罵�ͣ��ﵽ�Ż�����£����Ծݴ˽����⣮
��2�����������仯�������ͻ�ѧ�仯���ص���н��
��3�����ݻ���ױ�̿���ij̶�����������Ļ����¶ȣ�
��4��ȼ�յ���Ҫ��֮һ����ȼ������Ҫ���¶ȣ�������Ĺ����л���������ϵĴ���������ʹ���¶ȼ��罵�ͣ��ﵽ�Ż�����£����Ծݴ˽����⣮
����⣺��1������ʯ������ˮ�У�ʯ������ˮ�棬˵��ʯ�����ܶȱ�ˮ���ܶ�С��
��2����ȼ����ʱ���������仯���л�ѧ�仯����ȼ�����������ۻ������ɹ�̬��ΪҺ̬�������û�б仯��Ҳ����˵û�����������ɣ��ù������������仯��
��3��ȡһ�����סһ��Ѹ��ƽ���������Լ1���ȡ�����ɹ۲쵽���˱�ڣ��м������Ա仯�����ݻ�����˲������ȱ�ڣ�����˵���������������¶���ߣ�
��4��������Ĺ����п��������ӿ죬ȼ�շų�������Ѹ��ɢʧ��ʹ�¶ȵ���������Ż�㣬ȼ�ŵ���������Ϩ�𣬼�Cѡ�������
��ѡC��
�ʴ�Ϊ����1��С����2�������ۻ�����3�����棻��4��C��
��2����ȼ����ʱ���������仯���л�ѧ�仯����ȼ�����������ۻ������ɹ�̬��ΪҺ̬�������û�б仯��Ҳ����˵û�����������ɣ��ù������������仯��
��3��ȡһ�����סһ��Ѹ��ƽ���������Լ1���ȡ�����ɹ۲쵽���˱�ڣ��м������Ա仯�����ݻ�����˲������ȱ�ڣ�����˵���������������¶���ߣ�
��4��������Ĺ����п��������ӿ죬ȼ�շų�������Ѹ��ɢʧ��ʹ�¶ȵ���������Ż�㣬ȼ�ŵ���������Ϩ�𣬼�Cѡ�������
��ѡC��
�ʴ�Ϊ����1��С����2�������ۻ�����3�����棻��4��C��
������������Ҫ����ѧ����ʵ����������ͷ���������������Լ�ѧ����ʯ�������ʵ��˽⣬���ʱע����ϵ������Ϣ��ȷ����ʵ������

��ϰ��ϵ�д�
�����Ŀ
 ��2013?��������ͼ������غ��Ȼ��Ƶ��ܽ�����ͼ������ͼ��ش�
��2013?��������ͼ������غ��Ȼ��Ƶ��ܽ�����ͼ������ͼ��ش� ��2013?��������Ȼ���ж�����̼��ѭ��������ͼ��ʾ��
��2013?��������Ȼ���ж�����̼��ѭ��������ͼ��ʾ��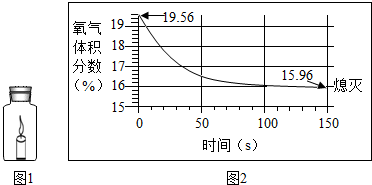 ��2013?�Ű�����������������ͼ1�ܱ�װ����ȼ����Ϩ�������������һ������ƿ�����������ı仯��ͼ2��ʾ�������ж���ȷ���ǣ�������
��2013?�Ű�����������������ͼ1�ܱ�װ����ȼ����Ϩ�������������һ������ƿ�����������ı仯��ͼ2��ʾ�������ж���ȷ���ǣ�������