��Ŀ����
��1���û�ѧ���ű�ʾ��3����ԭ�� ��+3�۵���Ԫ�� 1����������
��2���û�ѧʽ��ʾ���������е����ʣ�
�����ʯ��ˮ ������ˮ �� ���
��3�����������������Ľṹʾ��ͼ���ش��������⣺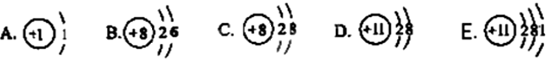
ͼ�������ܱ�ʾ ��Ԫ�أ�ͼ���ڻ�ѧ��Ӧ�����õ����ӵ�ԭ���� ������ţ���ͼ�б�ʾ�����ӵ��� ������ţ���ͼ�б�ʾ���������� �������ӷ��ţ���
��4���밴��Ҫ��д�����л�ѧ����ʽ��
��1���ó�������Ҫ�ɷ�����������������ԭ���� ��
��2����Ͱ����ʢ������ͭ��Һ��ԭ�� ��
��3��ʵ������ȡ������̼�� ��
��2���û�ѧʽ��ʾ���������е����ʣ�
�����ʯ��ˮ
��3�����������������Ľṹʾ��ͼ���ش��������⣺

ͼ�������ܱ�ʾ
��4���밴��Ҫ��д�����л�ѧ����ʽ��
��1���ó�������Ҫ�ɷ�����������������ԭ����
��2����Ͱ����ʢ������ͭ��Һ��ԭ��
��3��ʵ������ȡ������̼��
���������⿼�黯ѧ��������弰��д���ͻ�ѧ����ʽ����д������ؼ��Ƿ��廯ѧ����������Ķ����Ƿ��ӡ�ԭ�ӡ����ӻ��ǻ��ϼۣ������ڻ�ѧ����ǰ������λ�ü����ʵ��ļ������������ر��������壬���ܸ������ʻ�ѧʽ����д������ȷ��д���ʵĻ�ѧʽ����������ȷ�Ľ�������Ŀ��
����⣺��1������ԭ�ӡ�Ԫ�ػ��ϼۡ����ӵı�ʾ��������ʾ��3����ԭ�ӣ�3Fe����+3�۵���Ԫ�أ�
��1���������ӣ�Fe2+��
��2�����ݻ�ѧʽ�ı�ʾ�����������ʯ��ˮ������Ϊ�������ƣ��ʻ�ѧʽ��Ca��OH��2��������ˮ������Ϊ�Ȼ��ƣ��ʻ�ѧʽΪ��NaCl���Ƶ�����Ϊ�Ҵ����ʻ�ѧʽΪ��C2H5OH����Ƶ������ǵ⣬�ʻ�ѧʽ��I2��
��3����������������Ԫ�ص����࣬��������ͬ����ͬ��Ԫ�أ�����ͼ��ֻ������Ԫ�أ�һ����������������4�����ĵ��Ӷ��ﵽ�ȶ��ṹ����ΪA��ֻ��һ�����Ӳ㣬��һ��2�����ȶ��������õ�����AB�������ӵ����������ڵ���������������D�������ӵ�������С�ڵ��������������ӵ���C��
��4������ѧ����ʽ����дҪ����д��
���ó�������Ҫ�ɷ�����������������ԭ����3CO+Fe2O3
3CO2+2Fe��
����Ͱ����ʢ������ͭ��Һ��ԭ��������������ͭ��Һ������Ӧ��Fe+CuSO4=FeSO4+Cu��
��ʵ������ȡ������̼����ʯ��ʯ��ϡ���ᷴӦ��CaCO3+2HCl=CaCl2+H2O+CO2����
�𰸣���1��3Fe��
��Fe2+
��2��Ca��OH��2�� NaCl�� C2H5OH�� I2��
��3��3�� AB�� D�� C
��4����3CO+Fe2O3
3CO2+2Fe����Fe+CuSO4=FeSO4+Cu����CaCO3+2HCl=CaCl2+H2O+CO2����
| +3 |
| Fe |
��2�����ݻ�ѧʽ�ı�ʾ�����������ʯ��ˮ������Ϊ�������ƣ��ʻ�ѧʽ��Ca��OH��2��������ˮ������Ϊ�Ȼ��ƣ��ʻ�ѧʽΪ��NaCl���Ƶ�����Ϊ�Ҵ����ʻ�ѧʽΪ��C2H5OH����Ƶ������ǵ⣬�ʻ�ѧʽ��I2��
��3����������������Ԫ�ص����࣬��������ͬ����ͬ��Ԫ�أ�����ͼ��ֻ������Ԫ�أ�һ����������������4�����ĵ��Ӷ��ﵽ�ȶ��ṹ����ΪA��ֻ��һ�����Ӳ㣬��һ��2�����ȶ��������õ�����AB�������ӵ����������ڵ���������������D�������ӵ�������С�ڵ��������������ӵ���C��
��4������ѧ����ʽ����дҪ����д��
���ó�������Ҫ�ɷ�����������������ԭ����3CO+Fe2O3
| ||
����Ͱ����ʢ������ͭ��Һ��ԭ��������������ͭ��Һ������Ӧ��Fe+CuSO4=FeSO4+Cu��
��ʵ������ȡ������̼����ʯ��ʯ��ϡ���ᷴӦ��CaCO3+2HCl=CaCl2+H2O+CO2����
�𰸣���1��3Fe��
| +3 |
| Fe |
��2��Ca��OH��2�� NaCl�� C2H5OH�� I2��
��3��3�� AB�� D�� C
��4����3CO+Fe2O3
| ||
������������Ҫ����ѧ���Ի�ѧ�������д����������������ȫ�棬ע�ػ�������Ŀ�ѶȽ��ף�

��ϰ��ϵ�д�
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д�
�����Ŀ
��1���û�ѧ���ű�ʾ�������к������������� ���ؿ��к������Ľ���Ԫ���� ������ϸ������������������ Ԫ�أ�
��2�������������Ƶ�Ҫ��д����Ӧ�Ļ�ѧ����
��2�������������Ƶ�Ҫ��д����Ӧ�Ļ�ѧ����
| ���� | 1����ԭ�� | +3�۵���Ԫ�� | 5��ˮ���� | 2��þ���� |
| ��ѧ���� |
 ��2013?��ʯģ�⣩��1���û�ѧ���ű�ʾ��
��2013?��ʯģ�⣩��1���û�ѧ���ű�ʾ�� ��2013?��ʯģ�⣩��1���û�ѧ���ű�ʾ��
��2013?��ʯģ�⣩��1���û�ѧ���ű�ʾ��