��Ŀ����
����A~F�dz��л�ѧ�е�����ʵ��װ�ã��밴Ҫ����գ�
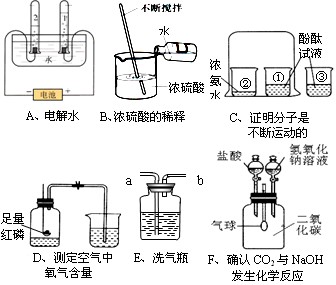
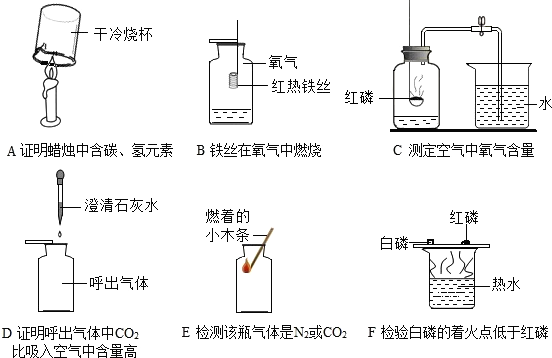
��1��Aʵ���Թ�2�в�����������________���Թ�1��2��������������ԼΪ________��
��2��Cʵ�����ձ��۵�������_______________��
��3��Dʵ��˵�����������Լռ������_______�����в���������__________��
�ټ��װ�������� ��ʵ��ǰ�н�ֹˮ�� ����ȴ���ٴ�ֹˮ�� ��ѡ�ý����ڵĿ���
��4������Eװ�ó�ȥO2�е�ˮ��������Һ���Լ�Ϊ________��ҽԺ���ô�װ�����۲�������������������______���a����b����Ӧ���Ӳ������������ܽ��ܡ�
��5��Fʵ���У�����ı仯�������_______����__________��д����������仯�Ļ�ѧ����ʽ____________��___________________��
��6������ʵ���в��ܴﵽʵ��Ŀ����______������ĸ����
��2��Cʵ�����ձ��۵�������_______________��
��3��Dʵ��˵�����������Լռ������_______�����в���������__________��
�ټ��װ�������� ��ʵ��ǰ�н�ֹˮ�� ����ȴ���ٴ�ֹˮ�� ��ѡ�ý����ڵĿ���
��4������Eװ�ó�ȥO2�е�ˮ��������Һ���Լ�Ϊ________��ҽԺ���ô�װ�����۲�������������������______���a����b����Ӧ���Ӳ������������ܽ��ܡ�
��5��Fʵ���У�����ı仯�������_______����__________��д����������仯�Ļ�ѧ����ʽ____________��___________________��
��6������ʵ���в��ܴﵽʵ��Ŀ����______������ĸ����
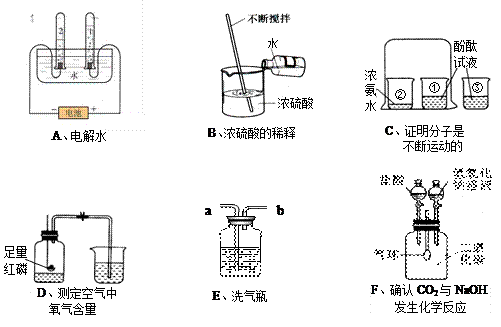
��1��������H2����1:2
��2�����ԱȻ�Ա�ʵ��
��3��1/5����
��4��Ũ���b
��5����������ͣ�����С����С�����CO2+2NaOH==Na2CO3+H2O��Na2CO3+2HCl==2NaCl+H2O+CO2��
��6��B
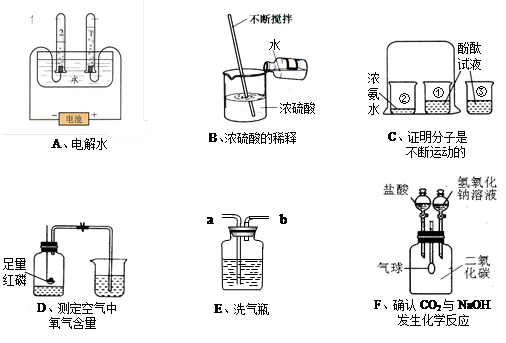
��2�����ԱȻ�Ա�ʵ��
��3��1/5����
��4��Ũ���b
��5����������ͣ�����С����С�����CO2+2NaOH==Na2CO3+H2O��Na2CO3+2HCl==2NaCl+H2O+CO2��
��6��B

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ