��Ŀ����
(2008��������)ij�����᳧�ų���β���к���SO2����ˮ�к���H2SO4 ����ش�
(1)���������꣬�����pH 5.6(�����������)��Ҫ���Եزⶨ����pH��ͨ���þ��� ��ֽ��
(2)���һ�������Σ���� ��
(3)����ʯ�Ҵ����ó���ˮ�Ļ�ѧ����ʽΪ�� ��
(4)�ó����������δ�ʩ������ (�����)��
A.����ѭ������ B.��������ȼ���ŷ�
C.��ˮ�ü��кͺ��ŷ� D.�Ľ����գ����ٷ�����ˮ�IJ���
(1)(2��)�� pH(��дΪPH��ph������) (2)(1��)Ӱ�����ཡ��(��ʹɭ�ִ���������������︯ʴ���������ữ�ȵȣ��������������)(3)(2��)Ca(OH)2+H2SO4==CaSO4+2H2O (4)(1��)B

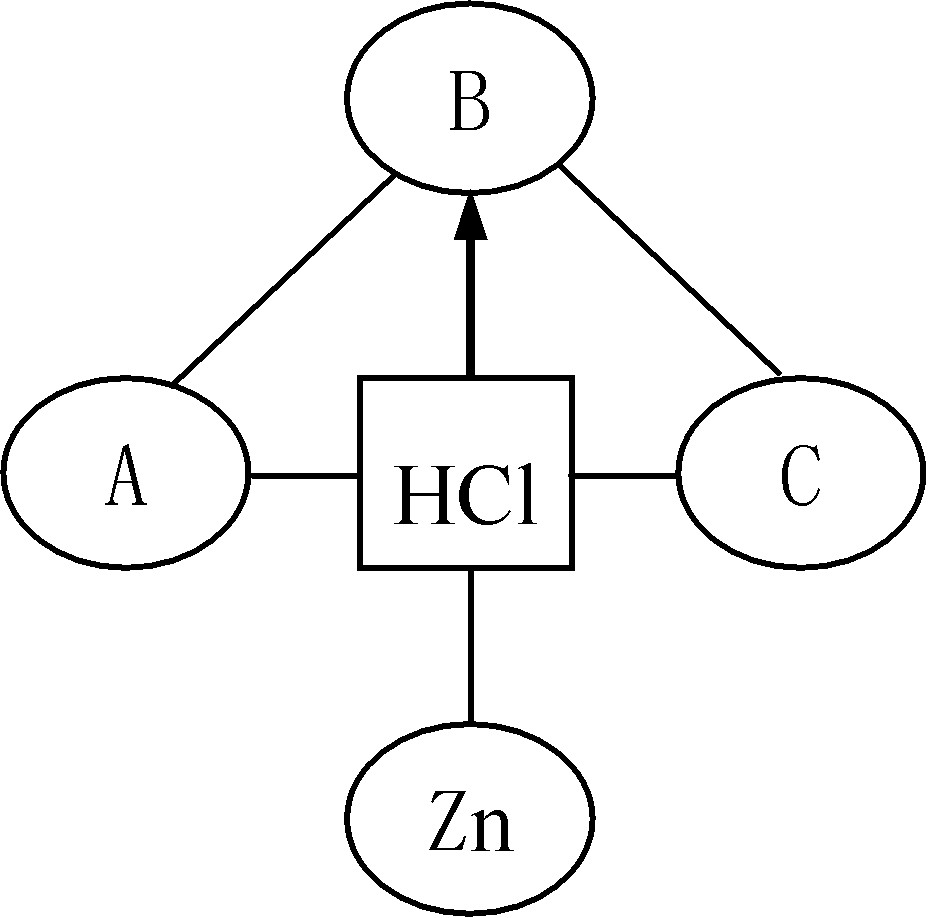
 ��������ϵ�д�
��������ϵ�д� ���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�߿�ϵ�д�(2008��̩����)С��ͬѧ��ʳƷ��װ���ڣ�������һ��Сֽ��������д�š���ʯ�Ҹ����������ʳ�á��������ֽ�Сֽ���ó������������ϣ�����һ�νϳ�ʱ�����ֽ���ڵİ�ɫ����ճ��һ���Ϊ��״����M����������һ��Կ�״����M����̽����
��̽������
(1)̽������M�Ƿ�ʧЧ��
(2)̽������M���Ƿ����������ơ�
��̽��������
��������� | ʵ�鲽�� | ʵ������ | ʵ����� |
̽��һ������M�Ƿ�ʧЧ | ȡ��������M������ʢ��ˮ���ձ��У����¶ȼƲ�������ǰ����¶� | �¶�û�����Ա仯 |
|
̽����������M���Ƿ����������� | �������ձ��ڵ�Һ���ֽ��衢���ã�ȡ�ϲ���Һ��������ɫ��̪��Һ |
| ���������� |
����˼��չ��
(1)����Ϊ��̽�������Ƿ����� ����ԭ���� ��
(2)����Ϊ����M�г��������������⣬�����ܺ��� ���������ʵ���Ƿ��и����� ��10.(2008��������)ʵ���ҳ��õĸ��������ʯ�ҡ���CaO����NaOH�Ļ��������������ˮ������CO2��Ӧ�����ʡ�ijͬѧ��һƿ���õġ���ʯ�ҡ���������̽����
(1)�����롿
�����û�б��ʣ�����ʯ�ҡ�ֻ����CaO������NaOH��
���������ȫ���ʣ�����ʯ�ҡ�ȫ�������CaCO3��Na2CO3��
��CaO��ˮ��Ӧ�ķ�Ӧ����ʽΪ ��
(2)��ʵ�顿����ͼ��ʾ��
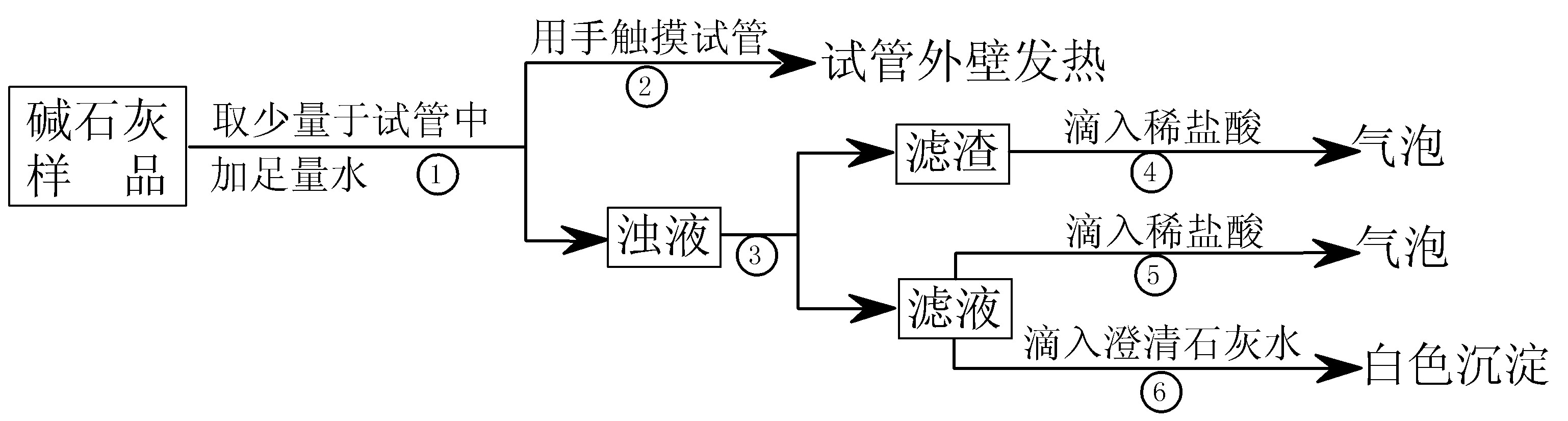
(3)���жϡ�
a.��Ca(OH)2��CaCO3��Na2CO3Ͷ�뵽ˮ�в�����ȣ����ɲ����������жϣ������ (�������������)
b.�����ܷ�����ѧ��Ӧ�ķ���ʽΪ ���ɲ����ݢ������жϣ���Һ�к��� (д��ѧʽ)���ɴ��жϲ���� (�������������)��
c.�ۺ�a��b�Ľ��ۣ��жϸ���Ʒ�������Ϊ ��
(4)����չ��������ʵ��˵����ʵ�����С���ʯ�ҡ�Ӧ ���棻�������в����������� ��