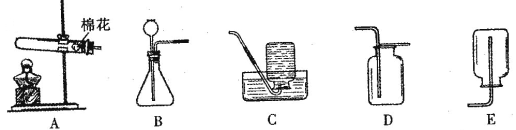
��Ŀ����
����Ŀ��(7��))����ʱ��ͬѧ�Dz����������ַ����ⶨij�Ȼ�����Һ����������������
��1������ѧ��������һ�����Ȼ�����Һ�м���������������Һ���õ�2.87g�Ȼ������壬����Ȼ�����Һ���Ȼ��Ƶ�����Ϊ���٣�(���ݻ�ѧ����ʽ��ʽ����)
�����ʵ��ⶨ������Һ��������������Ϊ10%��
��2��������������ȡһ��������Һ��������������ʵ���������£�
�����������(g) | 25.0 |
������+ʳ����Һ(g) | 45.0 |
������+ʳ�ξ���(g) | 27.4 |
���ݴ��� | ��Һ��������������Ϊ |
����ѧ�����ⶨ���ȷ�������������ⶨ�����������ԭ����
A������ʱδ�ò���������
B����ȡ�Ȼ�����Һ�����ϴ�
C������ʱ������������ʱ��ֹͣ����
D��ʵ���δ���������ϵİ�ɫ��������������
���𰸡���1�� 1.17g ��2�� 12%�� ����3�� C
��������
�����������1����һ�����Ȼ�����Һ�м���������������Һ��������Ӧ��NaCl+AgNO3==AgCl��+NaNO3
�����ݷ���ʽ��NaCl��AgCl��������ϵ����������Ȼ��Ƶ�����
�⣺�������Ȼ�������Ϊx
NaCl+AgNO3��AgCl�� +NaNO3
58.5 143.5
x 2.8 7g
![]()
x=1.17g
��2�������������������������+ʳ����Һ��������ɵó�ʳ����Һ������=45g-25g=20g�������������������������+ʳ�ξ����������ɵó�ʳ�ε�����=27.4g-25g=2.4g��������Һ��������������=2.4g/20g��100%=12%
��3��������ͨ�����������õ���ʳ�ε�����2.4g���ڻ�ѧ�����ⶨ���1.17g���������������ⶨ�����������ԭ���ǣ�A������ʱδ�ò��������裬Һ��ɽ�������ʳ�ε�����Ӧ���٣�����B����ȡ�Ȼ�����Һ�����ϴ�ֻӰ��������ʱ�䣬����C������ʱ������������ʱ��ֹͣ���ȣ������õ��ľ����оͺ���ˮ�֣�ʹ����ƫ��D��ʵ���δ���������ϵİ�ɫ��������������ֻ��ʹ�õ��ľ�����������

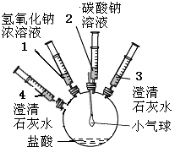
����Ŀ����11�֣�ijʵ��С���ͬѧҪ̽���������̼���ƵĻ�ѧ���ʣ����������ʵ��װ�ü�������
ʵ��װ�� | ʵ�鲽�輰���� | ʵ������ |
| ����ע����2�е���Һ����ʢ��ϡ�����ƿ�У������������ݲ����� | ������ |
����ע����3���������� | ����ʯ��ˮ����� | |
����ע����1�е���Һ����ƿ�� | ������� | |
�� | ���������� | |
����ע����4�е���Һ����ƿ�� |
��һ����1�����������������ԭ�� ��
��2�������������ɵó�̼���ƾ��� �����ʣ�
��3���������IJ����� ����Ŀ���� ��
��4���������з�����Ӧ�Ļ�ѧ����ʽ ��
��5�������ۢܢ��ɵó�̼���ƾ��� �����ʣ�
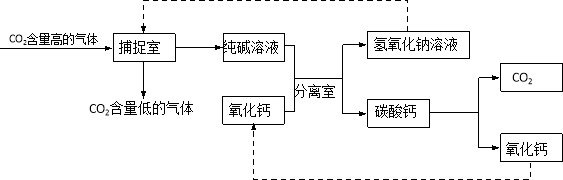
����������ʵ�������ʵ��С���ͬѧ�ֶ�ƿ����Һ�ijɷ�ʮ�ֺ��棬������̽����
��1����������롿ͨ��������һ����Ϊƿ����Һ��������һ������ �����ܺ����������ƻ� ��
��2����ʵ����֤�����ʵ�鷽��ȷ����Һ�����ʵ���ɣ�
ʵ����� | ʵ������ | ʵ����� |
�ֱ�ȡ������Һ��A��B��֧�Թ��У�A�� ����CaCl2��Һ��B�м���Na2CO3��Һ | A�в�����ɫ������B��û�г��� | ƿ����Һ�����ʵijɷ�Ϊ |
��3������˼�뽻����������ѧ��ѧ֪ʶ���������Ϸ����⣬������Щ���ʿ��Դ����Թ�B�м����Na2CO3��Һ��ɸ�ʵ�飿 ������ţ�
a.K2CO3 b.BaCO3 c.CO2 d.��ɫ��̪ e. CuSO4
����Ŀ�����������У�������ǿ����( )
ѡ �� | A | B | C | D |
���� | ���� | ����� | ���۾� | ��֭ |
pH | 8 | 2 | 12 | 3.5 |
A. A B. B C. C D. D