��Ŀ����
����Ŀ��ů�����еķ��ȼ������ۡ�ˮ��ʳ�εȣ����ȼ��ܴ�����ײⶨ������������������ʵ���ԭ������������������������װ�ü�ͼ��
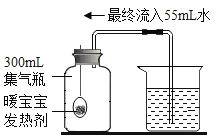
��1��ʵ������У��ձ���ˮ��ı仯��_________��
��2��ʵ��ʱ�ձ��е�ˮ������55mL����������������������Ϊ_________�����������С�����1λ����
��3����ʵ�����������������С��21%������Ϊԭ�������_____________��
��4���������ϵ�֪���������������������������Լ7%����ʱ�����ײ���ȼ�ա��ɴ˿�֪��ȼ�պ����ⶨ�������ȷ��ԭ����______________��
��5���øĽ�ʵ����ŵ���_______________��
���𰸡�ˮ���½� 18.3% װ��©��������ײ��㡢δ�ָ������¾ʹ��ɼеȣ� ����δ��ȫ���� ������ɻ�����Ⱦ���������ɣ�
��������
��1��ʵ������У�������٣���ѹ��С��С��������ѹ���ձ���ˮ��ı仯��ˮ���½���
��2��ʵ��ʱ�ձ��е�ˮ������55mL����������������������Ϊ![]() 18.3%��
18.3%��
��3����ʵ�����������������С��21%��ԭ�������װ��©������ײ��㡢δ�ָ������¾ʹ��ɼеȡ�
��4��ȼ�պ����ⶨ�������ȷ��ԭ��������δ��ȫ���ġ�
��5���øĽ�ʵ����ŵ��в�����ɻ�����Ⱦ���������ɣ���

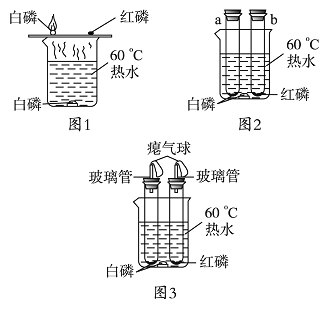
����Ŀ��ij��ȤС���У�ͬѧ�ǰ���ͼ1װ�ö�����ȼ��ȼ�յ�����������̽����̽�������У���Ҷ���ȼ�����ɵĴ��������Ƿ�Σ�����彡��������ʡ�
���������ϣ������Ż����40 �棬�����Ż����240 ��,ȼ�ղ��������������ǰ�ɫ���壬��̼�����������������������ˮ������Ӧ�������ж���ƫ����(HPO3)��
�����������ۣ����̶����彡���к�����ʵ��װ�ñ���Ľ���
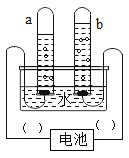
���Ľ���ʵ�飩ͬѧ�ǰ��Ľ����ͼ2װ�ý���ʵ�顣
����������ǽ��±�����������
���� | ���� |
a�Թ��а���ȼ�գ���ˮ�а���û��ȼ�ա� b�Թ��к���û��ȼ�ա� | ��ˮ�а��ס�b�Թ��к���û��ȼ�յ�ԭ��ֱ���_____��____�� |
����˼�����ۣ�(1)�Ľ����ͼ2װ����ͼ1װ�ñȽϣ��ŵ���_____��
(2)С��ͬѧָ��ͼ2װ�����в���֮�����������ͼ3װ�ã����������������_____��
(3)�ƾ�(C2H5OH)�ڿ�����ȼ�գ������������Ϊˮ�Ͷ�����̼,д����Ӧ�Ļ�ѧ����ʽ____��
����Ŀ����һ�������ļס��ҡ�����������������һ�ܱ������У���һ�������³�ַ�Ӧһ��ʱ���÷�Ӧ������ʵ����������ʾ�����ݱ�����Ϣ�ж�����˵����ȷ����
���� | �� | �� | �� | �� |
��Ӧǰ������/g | 4.0 | 2.0 | 3.0 | 2.0 |
��Ӧ�������/g | 1.2 | X | 2.4 | 5.4 |
A.X=2.0�����Ǵ���
B.�Ͷ��Ƿ�Ӧ��
C.�μӷ�Ӧ�ļס���������֮��Ϊ1��2
D.��һ���ǻ�����
����Ŀ���ں�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ�Ƶ��ص㡣�ס��ҡ���������ʾ�������ʣ����ǵIJ��ֻ�ѧʽ����ʾ��ͼ�ֱ������±���
���� | �� | �� | �� | �� | ͼ����
|
��ѧʽ | �� | C | H2O | H2 | |
��ʾ��ͼ |
|
|
| �� |
��1����д�������ʵĻ�ѧʽ_____�����������ʵ���ʾ��ͼ_____��
��2�����ֱ����ʻ�ѧ���ʵ�����_____��������д������
��3�������������������������_____���������ס��ҡ�����������