��Ŀ����
��5�֣�A��B��C��D��Ϊ���л�ѧ������������ᡢ����е�һ�����ʡ������ת����ϵ��ͼ��ͼ�С�������ʾ���ʼ����ת����ϵ����������ʾ���ʼ��ܷ�����ѧ��Ӧ����A��B��D�ж���������ϸ���к����������������ӵڶ�λ��Ԫ�أ�B��һ�ֳ������������壻D�㷺���ڲ�������ֽ����֯��ϴ�Ӽ���������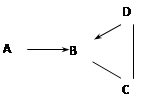
��1����д��A�Ļ�ѧʽ__________��
��2����д��B + C��Ӧ�Ļ�ѧ����ʽ________________________________________��
��3����д��C + D��Ӧ�Ļ�ѧ����ʽ________________________________________��
��1��H2CO3 ��2��Ca(OH)2 + CO2 CaCO3 ��+ H2O�������𰸼��ɣ�
��2��Ca(OH)2 + CO2 CaCO3 ��+ H2O�������𰸼��ɣ� ��3��Ca(OH)2 + Na2CO3 CaCO3��+ 2NaOH�������𰸼��ɣ�
��3��Ca(OH)2 + Na2CO3 CaCO3��+ 2NaOH�������𰸼��ɣ�
����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ