��Ŀ����
��1����֪ijԭ���еĵ��ӡ����ӡ����ӵ�������֮��Ϊ40������14�����Ӳ����磬���ԭ�ӵ����ԭ������Ϊ
��2��̼ԭ�ӵ�����Ϊ1.993��10-26Kg��12g̼Լ��
��3�������к��й�������ᣨC5H4N4O3��������ʹ�������ʯ������ʱ���Է������ʴ� ��C5H3N4OH�����������Ԫ�ص�����
��4�����»�����ײ������ʹ����立������ҵķֽⷴӦ�����ɴ��������岢�ų��������ȣ����������ը��2001��3��16��ʯ��ׯ��ը���У��ﷸ����������ըҩΣ������ΰ��ģ���֪����隣�ը��Ӧ��ѧ����ʽΪ��2NH4NO3�T2N2��+O2��+4X����X�Ļ�ѧʽ��
27
27
����2��̼ԭ�ӵ�����Ϊ1.993��10-26Kg��12g̼Լ��
6.02��1023
6.02��1023
��̼ԭ�ӣ���֪��ԭ�ӵ�����Ϊ9.288��10-26Kg������ԭ�ӵ����ԭ��������56
56
����3�������к��й�������ᣨC5H4N4O3��������ʹ�������ʯ������ʱ���Է������ʴ� ��C5H3N4OH�����������Ԫ�ص�����
��ͬ
��ͬ
�����ͬ����ͬ������ÿ�������к�����
��
ԭ�Ӹ�����ͬ����4�����»�����ײ������ʹ����立������ҵķֽⷴӦ�����ɴ��������岢�ų��������ȣ����������ը��2001��3��16��ʯ��ׯ��ը���У��ﷸ����������ըҩΣ������ΰ��ģ���֪����隣�ը��Ӧ��ѧ����ʽΪ��2NH4NO3�T2N2��+O2��+4X����X�Ļ�ѧʽ��
H2O
H2O
����������1������ԭ�ӵĽṹ�Լ�����ԭ�ӵ�����֮��Ĺ�ϵ���з��������ԭ��������������=������+��������
��2����һ��̼ԭ��������
Ϊ��������ԭ�ӵ�����������Ƚ����õ��ıȣ���Ϊ����ԭ�ӵ����ԭ��������
��3���������ᣨC5H4N4O3�������ʴ���C5H3N4OH����ѧʽ�ĺ�����з������
��4�����������غ㶨�ɿ�֪���ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû��������ԭ�ӵ�����Ҳû�иı䣮
��2����һ��̼ԭ��������
| 1 |
| 12 |
��3���������ᣨC5H4N4O3�������ʴ���C5H3N4OH����ѧʽ�ĺ�����з������
��4�����������غ㶨�ɿ�֪���ڻ�ѧ��Ӧ�У���Ӧǰ��ԭ�ӵ�����û�иı䣬��Ŀû��������ԭ�ӵ�����Ҳû�иı䣮
����⣺��1��ԭ��һ���������ӡ����ӡ����ӹ��ɵģ��������Ӳ����磬һ�����Ӵ�һ����λ������ɣ�һ�����Ӵ�һ����λ�ĸ���ɣ���ԭ���У�������=����������֪��Ԫ�ص�һ��ԭ����14���������磬Ҳ����˵��14�����ӣ����Ӻ͵��ӹ���40-14=26������Ϊ������=����������������Ϊ13���������ԭ��������������=13+14=27���ʴ�Ϊ��13��27��
��2��̼ԭ�ӵ�����Ϊ1.993��10-26Kg��12g̼Լ��̼ԭ�ӵĸ���Ϊ12g��1.993��10-26Kg=6.02��1023������ԭ�ӵ�����Ϊ9.288��10-26Kg������ԭ�ӵ����ԭ������=
��56���ʴ�Ϊ��6.02��1023��56��
��3�������ᣨC5H4N4O3�������ʴ���C5H3N4OH����ѧʽ��֪�����ᣨC5H4N4O3�������ʴ���C5H3N4OH��������̼���⡢����������Ԫ����ɵģ����������Ԫ�ص�������ͬ��
ÿ�����ᡢ���ʴ������о�����5��̼ԭ�ӡ�4����ԭ�Ӻ�4����ԭ�ӣ���1����������������ԭ����Ŀ�ֱ���3��1��ÿ�������к��е���ԭ����Ŀ��ͬ��
�ʴ�Ϊ����ͬ������
��4�����������غ㶨�ɺͻ�ѧ����ʽ��֪��X�Ļ�ѧʽ�к���NԪ�ص�ԭ�Ӹ���Ϊ����2��2-2��2����4=0������HԪ�ص�ԭ�Ӹ���Ϊ����2��4��4��=2������OԪ�ص�ԭ�Ӹ���Ϊ����2��3-2����4=1��
��X�Ļ�ѧʽΪ��H2O���ʴ�Ϊ��H2O��
��2��̼ԭ�ӵ�����Ϊ1.993��10-26Kg��12g̼Լ��̼ԭ�ӵĸ���Ϊ12g��1.993��10-26Kg=6.02��1023������ԭ�ӵ�����Ϊ9.288��10-26Kg������ԭ�ӵ����ԭ������=
| 9.288��10-26Kg | ||
1.993��10-26Kg��
|
��3�������ᣨC5H4N4O3�������ʴ���C5H3N4OH����ѧʽ��֪�����ᣨC5H4N4O3�������ʴ���C5H3N4OH��������̼���⡢����������Ԫ����ɵģ����������Ԫ�ص�������ͬ��
ÿ�����ᡢ���ʴ������о�����5��̼ԭ�ӡ�4����ԭ�Ӻ�4����ԭ�ӣ���1����������������ԭ����Ŀ�ֱ���3��1��ÿ�������к��е���ԭ����Ŀ��ͬ��
�ʴ�Ϊ����ͬ������
��4�����������غ㶨�ɺͻ�ѧ����ʽ��֪��X�Ļ�ѧʽ�к���NԪ�ص�ԭ�Ӹ���Ϊ����2��2-2��2����4=0������HԪ�ص�ԭ�Ӹ���Ϊ����2��4��4��=2������OԪ�ص�ԭ�Ӹ���Ϊ����2��3-2����4=1��
��X�Ļ�ѧʽΪ��H2O���ʴ�Ϊ��H2O��
�����������ѶȽϴ�����ԭ���е��й�������ϵ�����ԭ�������ļ����Լ������غ㶨�ɵ��й�֪ʶ�ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ
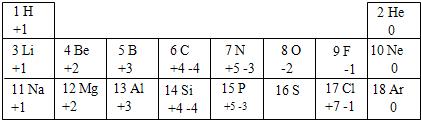
������Χ��������������100����Ԫ����ɵģ�Ϊ�˱����о�Ԫ�ص����ʣ�������ҪѰ������֮������ڹ��ɣ��±��г�����1��18��Ԫ�صIJ�����������ϼۺ�������ϼۣ������Ķ��ش��������⣺
| 1 H +1 | 2 He 0 | ||||||
| 3 Li +1 | 4 Be +2 | 5 B +3 | 6 C +4-4 | 7 N +5-3 | 8 O -2 | 9 F -1 | 10 Ne 0 |
| 11 Na +1 | 12 Mg +2 | 13 Al +3 | 14 Si +4-4 | 15 P +5-3 | 16 S | 17 Cl +7-1 | 18 Ar 0 |
 �����Ԫ�ص�________������������Ϊ________������________Ԫ�أ���������ǽ���������11��Ԫ���ڻ�ѧ��Ӧ���γɵ�������________��дԪ�ط��ţ�ԭ�Ӿ�����ͬ�ĺ�������Ų���
�����Ԫ�ص�________������������Ϊ________������________Ԫ�أ���������ǽ���������11��Ԫ���ڻ�ѧ��Ӧ���γɵ�������________��дԪ�ط��ţ�ԭ�Ӿ�����ͬ�ĺ�������Ų�����2���Ʋ�16��Ԫ�ص�������ϼ�Ϊ________����������ϼ۵�������Ļ�ѧʽΪ________��
��3��Ԫ�����ڱ�������ѧϰ��ѧ����Ҫ���ߣ��������źܶ���ɣ��ӻ��ϼ۽Ƕȷ��������ǿ���˵�����е�һ�����ɣ�________��
��2008?�Ϻ���ģ�⣩������Χ��������������100����Ԫ����ɵģ�Ϊ�˱����о�Ԫ�ص����ʣ�������ҪѰ������֮������ڹ��ɣ��±��г�����1��18��Ԫ�صIJ�����������ϼۺ�������ϼۣ������Ķ��ش��������⣺
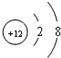
��1����֪ijԪ��R2+�����ӽṹʾ��ͼΪ �����Ԫ�ص�______������������Ϊ______������______Ԫ�أ���������ǽ���������11��Ԫ���ڻ�ѧ��Ӧ���γɵ�������______��дԪ�ط��ţ�ԭ�Ӿ�����ͬ�ĺ�������Ų���
�����Ԫ�ص�______������������Ϊ______������______Ԫ�أ���������ǽ���������11��Ԫ���ڻ�ѧ��Ӧ���γɵ�������______��дԪ�ط��ţ�ԭ�Ӿ�����ͬ�ĺ�������Ų���
��2���Ʋ�16��Ԫ�ص�������ϼ�Ϊ______����������ϼ۵�������Ļ�ѧʽΪ______��
��3��Ԫ�����ڱ�������ѧϰ��ѧ����Ҫ���ߣ��������źܶ���ɣ��ӻ��ϼ۽Ƕȷ��������ǿ���˵�����е�һ�����ɣ�______��
| 1 H +1 | 2 He | ||||||
| 3 Li +1 | 4 Be +2 | 5 B +3 | 6 C +4-4 | 7 N +5-3 | 8 O -2 | 9 F -1 | 10 Ne |
| 11 Na +1 | 12 Mg +2 | 13 Al +3 | 14 Si +4-4 | 15 P +5-3 | 16 S | 17 Cl +7-1 | 18 Ar |
 �����Ԫ�ص�______������������Ϊ______������______Ԫ�أ���������ǽ���������11��Ԫ���ڻ�ѧ��Ӧ���γɵ�������______��дԪ�ط��ţ�ԭ�Ӿ�����ͬ�ĺ�������Ų���
�����Ԫ�ص�______������������Ϊ______������______Ԫ�أ���������ǽ���������11��Ԫ���ڻ�ѧ��Ӧ���γɵ�������______��дԪ�ط��ţ�ԭ�Ӿ�����ͬ�ĺ�������Ų�����2���Ʋ�16��Ԫ�ص�������ϼ�Ϊ______����������ϼ۵�������Ļ�ѧʽΪ______��
��3��Ԫ�����ڱ�������ѧϰ��ѧ����Ҫ���ߣ��������źܶ���ɣ��ӻ��ϼ۽Ƕȷ��������ǿ���˵�����е�һ�����ɣ�______��
 �����Ԫ�ص�
�����Ԫ�ص�