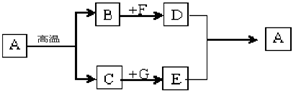
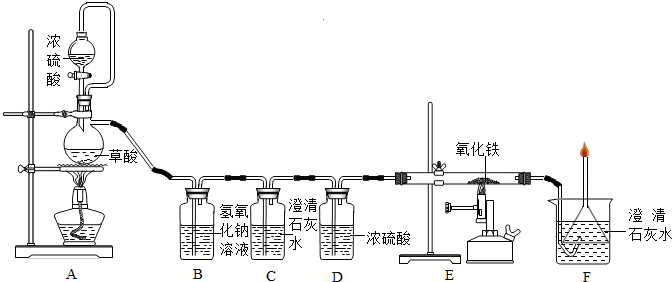
��Ŀ����
������ʹ�����Ľ������ϡ�
��1�������ֶ������Ͻ����к�̼���ϸߵ���_______________��
��2�����dz��á�ͭǽ���ڡ�����������ļ�̡�������һ��������Ҳ�ܷ������ַ�Ӧ������˿��������ȼ�գ���Ӧ�Ļ�ѧ����ʽ��_________________________________________��
��3����m g����ͭ����ϡ��������ȫ�ܽ���ټ������۳�ַ�Ӧ�����ˣ��õ�����A����ҺB���ٽ�����A��������ϡ�����У�������ð������ַ�Ӧ��ʣ��������ʵ�����Ϊ12.8 g��
�������ijɷ���__________����ҺB�ijɷ���_________________��
��ԭ����ͭ������Ϊ________g��
��1������ ��2��3Fe��2O2 Fe3O4 ��3����Cu��Fe FeSO4��H2O ��16
Fe3O4 ��3����Cu��Fe FeSO4��H2O ��16
��������1�������ĺ�̼��Ϊ2%��4.3%���ֵĺ�̼��Ϊ0.03%��2%����2������������ȼ��������������������3������A�м���ϡ����������ð�����������к����������۽�����ͭ��Һ�е�ͭȫ���û���������ʣ�࣬����A����ϡ���ᷴӦ��ȫ֮��ʣ�������ȫ��ͭ����ͭ�����������ԭ����ͭ��������

��ϰ��ϵ�д�
�����Ŀ
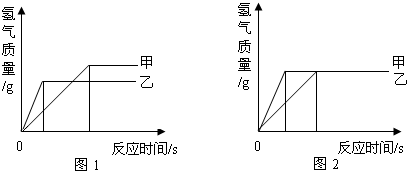
 ������ʹ�����Ľ������ϣ�
������ʹ�����Ľ������ϣ�