��Ŀ����
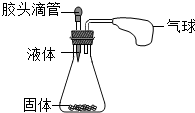 ��2012?�����ж�ģ����ͼ��ʾ����ѹ��ͷ�ι��е�Һ�壬ʹ֮��ƿ�й���Ӵ�����ʹС�������������д����������Ҫ��Ļ�ѧ����ʽ��
��2012?�����ж�ģ����ͼ��ʾ����ѹ��ͷ�ι��е�Һ�壬ʹ֮��ƿ�й���Ӵ�����ʹС�������������д����������Ҫ��Ļ�ѧ����ʽ����1���ֽⷴӦ
2H2O2
2H2O+O2��
| ||
2H2O2
2H2O+O2��
��
| ||
��2�����Ϸ�Ӧ
CaO+H2O�TCa��OH��2
CaO+H2O�TCa��OH��2
����3���û���Ӧ
Fe+2HCl=FeCl2+H2��
Fe+2HCl=FeCl2+H2��
����4�����ֽⷴӦ
CaCO3+2HCl=CaCl2+H2O+CO2��
CaCO3+2HCl=CaCl2+H2O+CO2��
������������װ�������������Ϊ��ƿ��ѹǿ����ԭ���Ƿ�Ӧ���Ȼ��������������ٳ�����Ҫ������ӣ�
����⣺��1����������ֽ�ʱ��ų������������ι����ǹ������⣬��ƿ���Ƕ������̣����Ҷ��߷����ķ�Ӧ�ǷֽⷴӦ���ʷ���ʽΪ��2H2O2
2H2O+O2����
��2��CaO��ˮ��Ӧ�ų��������ȣ�����Һ���¶����ߣ�ʹ�������ͣ������÷�ӦΪ���Ϸ�Ӧ���ʷ���ʽΪ��CaO+H2O�TCa��OH��2��
��3���������ᷢ�����û���Ӧ�ܲ����������������磺Fe+2HCl=FeCl2+H2����
��4��ʯ��ʯ��ϡ���ᷢ�����ֽⷴӦ����������̼����ʹ�����÷�Ӧ�ķ���ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2��
�ʴ�Ϊ����1��2H2O2
2H2O+O2����
��2��CaO+H2O�TCa��OH��2
��3��Fe+2HCl=FeCl2+H2��
��4��CaCO3+2HCl=CaCl2+H2O+CO2�����𰸺������ɣ�
| ||
��2��CaO��ˮ��Ӧ�ų��������ȣ�����Һ���¶����ߣ�ʹ�������ͣ������÷�ӦΪ���Ϸ�Ӧ���ʷ���ʽΪ��CaO+H2O�TCa��OH��2��
��3���������ᷢ�����û���Ӧ�ܲ����������������磺Fe+2HCl=FeCl2+H2����
��4��ʯ��ʯ��ϡ���ᷢ�����ֽⷴӦ����������̼����ʹ�����÷�Ӧ�ķ���ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2��
�ʴ�Ϊ����1��2H2O2
| ||
��2��CaO+H2O�TCa��OH��2
��3��Fe+2HCl=FeCl2+H2��
��4��CaCO3+2HCl=CaCl2+H2O+CO2�����𰸺������ɣ�
������������һ�������Ե����⣬�����˸��ݶԷ�Ӧ�����̽��������ѧ��Ӧ��ѹǿԭ�����ϣ�������ѧ����ʵ��װ�õĴ��¡�ʵ������ķ�����ʵ�鷽������Ƶ�������

��ϰ��ϵ�д�
 ѧҵ����һ��һ��ϵ�д�
ѧҵ����һ��һ��ϵ�д�
�����Ŀ
 ��2012?�����ж�ģ��Ϊ�˲ⶨij�ֻ�ͭ��ͭ��п�ĺϽ𣩵���ɣ�ȡ�û�ͭ��Ʒ��м100g����400gϡ�����4�μ��뵽����Ʒ�У�������ݼ�¼���±���
��2012?�����ж�ģ��Ϊ�˲ⶨij�ֻ�ͭ��ͭ��п�ĺϽ𣩵���ɣ�ȡ�û�ͭ��Ʒ��м100g����400gϡ�����4�μ��뵽����Ʒ�У�������ݼ�¼���±���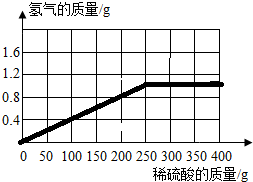
 ��2012?�����ж�ģ����֪�������ԭ�ӽṹʾ��ͼ���£�
��2012?�����ж�ģ����֪�������ԭ�ӽṹʾ��ͼ���£�