��Ŀ����
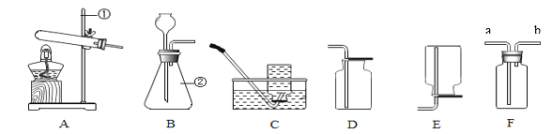
����Ŀ���⻯þ����ѧʽΪMgH2����һ�ֳ��õ�������ij��ȤС����ѡ������ͼװ���Ʊ��⻯þ��
���������ϣ�
��1���⻯þ��������������þ�ۼ����Ƶá��⻯þ��ˮ��Ӧ����������þ[Mg(OH)2]�����������ų�������

��2��������þ����ˮ��Ӧ����״�����������ܶ�Ϊ0.09g/L��
��ʵ�鲽�裩
��������װ�ã��١�����
��װ��ҩƷ����Һ©��������װ����ͨ��������D���ռ��������鴿��Cװ�ü��ȡ�����ͨ��ƽ�ȵ���������
��ʵ�����ʱ����ֹͣ���ȣ���װ����ȴ������ֹͣͨ��������
�ش��������⣺
��1���뽫������еIJ�����������____��
��2��װ��A�з�Ӧ�Ļ�ѧ����ʽ____��A���÷�Һ©������ϡ������ŵ���____���Ƶõ��⻯þ����____���档
��3��ʵ�鿪ʼʱҪ����ͨ��������Ŀ�ģ�����ֹ���������ȵĿ�������ը�⣬����____��Bװ�õ�������____��
��4��ʵ�����ͬѧ��������ͼװ�ü��õ����⻯þ�Ĵ��ȡ�ȡһ��������Ʒ����ͼ����Y�ι�һ�ˣ�ʵ�鿪ʼ��Y�ι���б��ˮ����Ʒ�Ӵ������ռ�������178ml������Ʒ���⻯þ��������____g��
��5����������Ľ��ƫ���ܵ�ԭ����____��
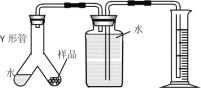
A��Y�ι�����ˮ�����Ҳ��ų���������
B������������Ͳ̫����δ�������ó��Ͷ���
C��δ��ȴ�����¾Ͷ���
D��ʵ��ǰY�ι����п���
���𰸡����װ�õ������� Zn+H2SO4=ZnSO2+H2 �� ���Կ���Һ��ҩƷ���������Ʒ�Ӧ������ �ܷ� ��ֹ�ȼ���þ��������е����巢����Ӧ �������� 0.1g BC
��������
��1�����й�����ǰ��Ҫ������װ�ã��ټ��װ�õ���������
��2��ϡ�����п����Ӧ��ȡ��������ѧ����ʽΪ��Zn+H2SO4=ZnSO4+H2�����÷�Һ©������ϡ������ŵ��ǿ��Կ���Һ��ҩƷ���������Ʒ�Ӧ�����ʣ��⻯þ��ˮ��Ӧ����������þ[Mg(OH)2]�����������ų������������ܷⱣ�棻
��3�����ȹ����У������������п����ᷢ����ը��þ����������Ӧ��������þ��ʵ�鿪ʼʱҪ����ͨ��������Ŀ���Ƿ�ֹ�ȼ���þ��������е����巢����Ӧ�����������ȵĿ�������ը��Bװ�õĸ�������������������е�ˮ����ֹˮ��þ��Ӧ��
��4������m=��V�ɼ��������������Ϊ0.09mg/mL��178.0mL=16.02mg�����ݷ���ʽMgH2+2H2O=Mg��OH��2+2H2������֪�⻯þ������Ϊ![]() ��16.02mg=104mg=0.1g��
��16.02mg=104mg=0.1g��
��5��A��Y�ι�����ˮ�����Ҳ��ų��������壬����Ӱ���ռ��������������������䣬�ʴ���
B������������Ͳ̫����δ�������ó��Ͷ����������ռ������������ƫ���ƫ����ȷ��
C��δ��ȴ�����¾Ͷ����������ռ������������ƫ���ƫ����ȷ��
D��ʵ��ǰY�ι����п���������Ӱ���ռ��������������������䣬�ʴ�����

����Ŀ����ʯͷֽ��������ĥ�ɷ�ĩ��ʯͷΪ��Ҫԭ�����ɵġ�����ֽ��ˮ��̲���ȼ�գ�����Ҫ���Dz��ÿ�����ֽ���dz��������ճ̱�����ǩֽ�ȶ���������̼���Ϊ��Ҫԭ�ϵĵ�̼��ʯͷֽ����Ϊ�ⶨ����̼��Ƶĺ���������С���ͬѧ��ȡ50g��ֽ��Ʒ���ֱ���5ֻ�ձ��н�����ʵ�飬ʵ�����ݼ��±�������ֽ�������ɷּȲ�����ˮ��Ҳ����ˮ�����ᷴӦ����
�ձ��� | �ձ��� | �ձ��� | �ձ��� | �ձ��� | |
������Ʒ������/g | 10 | 10 | 10 | 10 | 10 |
����ϡ���������/g | 10 | 20 | 30 | 40 | 50 |
��ַ�Ӧ���������������/g | 0.88 | 1.76 | X | 3.52 | 3.52 |
��1������X��ֵΪ________��
��2�����ձ�_______��̼�����ȫ��Ӧ��
��3������Ʒ��̼��Ƶ���������_____��