��Ŀ����
ʵ������ȡ���������װ������ͼ��ʾ��

��1������a��������________��
��2��A��E���ӿ���ȡO2����Ӧ�Ļ�ѧ����ʽ��________________________��ѡ��E�ռ�O2��ԭ����________________________����������ص�ʵ����������У���ȷ����________����������ţ���
���ȼ��װ�õ������ԣ��ټ���ҩƷ
�ڼ���ʱ����ʹ�Թܾ������ȣ��ٶ���ҩƷ��λ���м���
�۴����ܿڳ��������������ݺ��ٽ��������뼯��ƿ�н����ռ�
��ʵ�����ʱ����Ϩ��ƾ��ƣ��ٽ����ܴ�ˮ����ȡ��
��3��B��C����Ҳ����ȡO2����Ӧ�Ļ�ѧ����ʽ��________________________��
��4��B��C���ӻ�����ȡCO2����Ӧ�Ļ�ѧ����ʽ��________________________��
����ȼ�ŵ�ľ�����ڼ���ƿƿ�ڣ��۲쵽________________��˵��ƿ���ѳ���CO2������CO2ͨ�����ʯ��ˮ�У��۲쵽��������________________���÷�Ӧ�Ļ�ѧ����ʽ��________________________��

��1������a��������________��
��2��A��E���ӿ���ȡO2����Ӧ�Ļ�ѧ����ʽ��________________________��ѡ��E�ռ�O2��ԭ����________________________����������ص�ʵ����������У���ȷ����________����������ţ���
���ȼ��װ�õ������ԣ��ټ���ҩƷ
�ڼ���ʱ����ʹ�Թܾ������ȣ��ٶ���ҩƷ��λ���м���
�۴����ܿڳ��������������ݺ��ٽ��������뼯��ƿ�н����ռ�
��ʵ�����ʱ����Ϩ��ƾ��ƣ��ٽ����ܴ�ˮ����ȡ��
��3��B��C����Ҳ����ȡO2����Ӧ�Ļ�ѧ����ʽ��________________________��
��4��B��C���ӻ�����ȡCO2����Ӧ�Ļ�ѧ����ʽ��________________________��
����ȼ�ŵ�ľ�����ڼ���ƿƿ�ڣ��۲쵽________________��˵��ƿ���ѳ���CO2������CO2ͨ�����ʯ��ˮ�У��۲쵽��������________________���÷�Ӧ�Ļ�ѧ����ʽ��________________________��
��1���ƾ���
��2��2KMnO4��K2MnO4 +MnO2 + O2���� ������������ˮ�Ҳ���ˮ��Ӧ
�٢ڢ�
��3��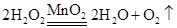
 ľ��Ϩ�� ����ʯ��ˮ�����
ľ��Ϩ�� ����ʯ��ˮ����� 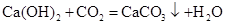
��2��2KMnO4��K2MnO4 +MnO2 + O2���� ������������ˮ�Ҳ���ˮ��Ӧ
�٢ڢ�
��3��
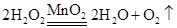
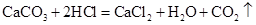 ľ��Ϩ�� ����ʯ��ˮ�����
ľ��Ϩ�� ����ʯ��ˮ����� 
�����������2��A��E���ӿ���ȡO2�����õ��ǹ̹̼����ͣ��Թܿ���һ�����������ø��������ȡ�������������ܶȱȿ������������ſ������ռ�������������ˮ�����ɲ�����ˮ���ռ���Eװ�õ��ռ���������ˮ���ռ������ȸ�����طֽ���ȡ������ͬʱ��������������غͶ������̣��ʷ�Ӧ�Ļ�ѧ����ʽ��2KMnO4��K2MnO4 +MnO2 + O2�����ռ������Ժ�Ҫ�Ƚ����ܴ�ˮ����ȡ������ֹͣ���ȣ�����ˮ�������Թ���ʹ�Թ����ѡ�
��3��B��C������ȡO2�����ڹ�Һ�峣���µķ�Ӧ�������ù���������ȡ������ʵ������ʯ��ʯ��ϡ���ᷴӦ��ȡ������̼���壺CaCO3+2HCl===CaCl2+H2O+CO2����������̼���ܶȱȿ�����������ˮ�����������ſ������ռ������ڶ�����̼����֧��ȼ�գ��������ķ����ǽ�ȼ�ŵ�ľ�����ڼ���ƿ�ڣ���Ϩ���ʾ���ռ����ˣ�������̼�ļ��������ö�����̼��ʹ�����ʯ��ˮ����ǡ�

��ϰ��ϵ�д�
�����Ŀ

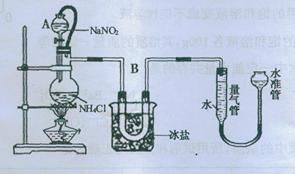
 NaCl+N2��+2H2O
NaCl+N2��+2H2O






