��Ŀ����
��2010?��������ģ��������һ�������Դ���⣬���ǿ��ԴӺ�ˮ����ȡ�Ȼ��ƣ�ijͬѧ���������̴Ӻ�����ɳ���Ȼ��Ƶ�ʳ��ˮ����ȡ20g���������Ȼ��ƵĴ��Σ����ⶨ�ô������Ȼ��Ƶ�������������Ҫ�������̣�����Ҫ��Ӧ��CaCl2+Na2CO3��CaCO3��+2NaCl��
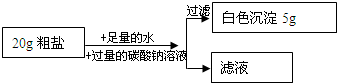
��1�������ǽ��������з��룬������������������������
��2��20g�������Ȼ��Ƶ����ʵ���Ϊ
��3���ô������Ȼ��Ƶ���������Ϊ

��1�������ǽ��������з��룬������������������������
���岻������Һ��
���岻������Һ��
����2��20g�������Ȼ��Ƶ����ʵ���Ϊ
0.05mol
0.05mol
����3���ô������Ȼ��Ƶ���������Ϊ
72.25%
72.25%
����������1�������Ƿ��벻���Թ����Һ���һ�ֳ��õķ�����
��2���������ɳ�����������ϻ�ѧ����ʽ����������Ȼ��Ƶ�����������������Ȼ��Ƶ����ʵ�����
��3�����ݣ�2���м���������������Ȼ��Ƶ�������������ʵ����������ļ��㹫ʽ��������Ȼ��Ƶ�����������
��2���������ɳ�����������ϻ�ѧ����ʽ����������Ȼ��Ƶ�����������������Ȼ��Ƶ����ʵ�����
��3�����ݣ�2���м���������������Ȼ��Ƶ�������������ʵ����������ļ��㹫ʽ��������Ȼ��Ƶ�����������
����⣺��1�������Ƿ��벻���Թ����Һ���һ�ֳ��õķ����������Ա��������ʱ��벻������Һ�壻
��2������ͼ����ʾ����֪��������5g̼��ƣ�����̼��Ƶ����ʵ���Ϊ��5g��100g/mol=0.05mol���ʣ�
��20g�������Ȼ��Ƶ����ʵ���Ϊx����
CaCl2+Na2CO3��CaCO3��+2NaCl
1 1
x 0.05mol
=
��ã�x=0.05mol��
��3���ô������Ȼ��Ƶ���������Ϊ��
��100%=72.25%��
�𣺣�2��20g�������Ȼ��Ƶ����ʵ���Ϊ0.05mol����3���ô������Ȼ��Ƶ���������Ϊ72.25%��
��2������ͼ����ʾ����֪��������5g̼��ƣ�����̼��Ƶ����ʵ���Ϊ��5g��100g/mol=0.05mol���ʣ�
��20g�������Ȼ��Ƶ����ʵ���Ϊx����
CaCl2+Na2CO3��CaCO3��+2NaCl
1 1
x 0.05mol
| 1 |
| x |
| 1 |
| 0.05mol |
��ã�x=0.05mol��
��3���ô������Ȼ��Ƶ���������Ϊ��
| 20g-0.05mol��111g/mol |
| 20g |
�𣺣�2��20g�������Ȼ��Ƶ����ʵ���Ϊ0.05mol����3���ô������Ȼ��Ƶ���������Ϊ72.25%��
������������Ҫ���麬�������ʵĻ�ѧ����ʽ������������������ļ��㣬�ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ