��Ŀ����
����Ŀ���Ȼ�����һ����Ҫ�Ļ���ԭ�ϡ�����Ȼ�����Һ���Ƶ��������������ƺ���������Ӧ�Ļ�ѧ����ʽΪ2NaCl+2H2O![]() Cl2��+H2��+2NaOH����ȡһ���������Ȼ�����Һ���е�⣬���Ȼ�����ȫ��Ӧʱ���õ�85.4g��Һ������������������ʱ��Ĺ�ϵ��ͼ��ʾ�������:
Cl2��+H2��+2NaOH����ȡһ���������Ȼ�����Һ���е�⣬���Ȼ�����ȫ��Ӧʱ���õ�85.4g��Һ������������������ʱ��Ĺ�ϵ��ͼ��ʾ�������:
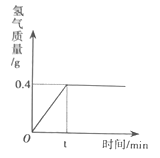
(1)�Ȼ�����ȫ��Ӧʱ����������������_________g��
(2)�Ȼ�����ȫ��Ӧʱ�������������Ƶ�������_________��?
(3)ԭ�Ȼ�����Һ�����ʵ�����������_________?
���𰸡� 0.4 16 23.4%
�����������ݷ�Ӧ�Ļ�ѧ����ʽ�����ṩ�����ݼ���������1����ͼ��֪���Ȼ�����ȫ��Ӧʱ������������������0.4g����2���������������Ƶ�������x����������������Ϊy���Ȼ��Ƶ�����Ϊz��
2NaCl+2H2O![]() Cl2��+H2��+2NaOH
Cl2��+H2��+2NaOH
117 71 2 80
z y 0.4g x
![]()
x=16g
y=14.2g
z=23.4g
��3��ԭ�Ȼ�����Һ�����ʵ���������Ϊ![]() ��100�� =23.4%
��100�� =23.4%
��(2)�Ȼ�����ȫ��Ӧʱ�������������Ƶ�������16����(3)ԭ�Ȼ�����Һ�����ʵ�����������23.4%��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
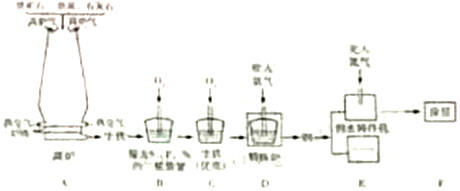
Сѧ��10����Ӧ����ϵ�д�����Ŀ���ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ�����ᷢչ����������˾��ס��ϳɰ��Ĺ�������ͼ���£�
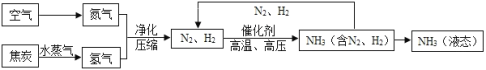
(1)���������ɵİ�(NH3)����ũҵ���Ǻϳ�_________(���������������������ط���)��ԭ�ϡ�
(2)��ȡ�����ķ�Ӧ����Ϊ��C + H2O![]() CO + H2�����з�����ԭ��Ӧ��������_____________��
CO + H2�����з�����ԭ��Ӧ��������_____________��
(3)д��N2��H2��Ӧ����NH3�Ļ�ѧ����ʽ_________________________________��
(4)�������п�ѭ��ʹ�õ�������___________________��
(5)���е㲻ͬ��������뿪����������Һ�����뷨���±��Ǹ����ʵķе㡣
���� | H2 | N2 | O2 | NH3 |
�е� | �C 252�� | �C 195.8�� | �C 183�� | �C 33.35�� |
������¶�t��![]() ʱ���ɽ�������N2��O2���롣Ҫ������NH3��N2��H2���뿪����Ӧ���¶ȿ�����_________(�����)��
ʱ���ɽ�������N2��O2���롣Ҫ������NH3��N2��H2���뿪����Ӧ���¶ȿ�����_________(�����)��
A��![]() B��
B��![]() C��
C��![]()
����Ŀ��С������H2O2��Һ��O2��ʵ��̽����������й��̣��ش��й����⡣
��1��MnO2����������5mL5%��H2O2��Һ�м�������MnO2�����������������ݡ�
��д����H2O2��Һ�Ʊ�O2�Ļ�ѧ����ʽ��____________________________________��
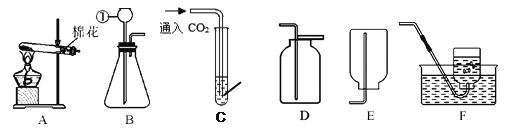
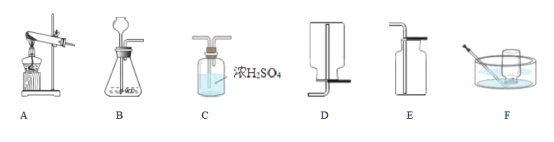
����������Ӧԭ���Ʊ����ռ�һƿ�����O2��������װ��ͼ��ѡ����װһ��װ�ã�������˳��Ϊ_______�� __________�� ________�����ţ���Ϊ��ȷ��ʵ��ɹ�����װҩƷ֮ǰӦ��_________��
�ۼ���O2�ķ�����___________________________ ����ľ����ȼ����֤���ռ�������ΪO2��
��2��FeCl3��Һ������
��5mL5%��H2O2��Һ�м���2��һ��Ũ�ȵ� FeCl3��Һ�����������������ݡ�
����֪��FeCl3��Һ����Ҫ������������H2O��Fe3+��Cl-
�����⣩��������H2O2��Һ�ķֽ�������ã�
�����裩����һ��������H2O
�������������Fe3+
��������������Cl-
���������ټ���һ�����ܳ�����������__________________________________________��
��ʵ�飩
���� | ���� |
�����������䣬��H2O2��Һ�м���NaCl��Һ | �����Ա仯 |
�����������䣬��H2O2��Һ�м���Na2SO4��Һ | �����Ա仯 |
�����������䣬��H2O2��Һ�м���Fe2(SO4)3��Һ | ���������������� |
�����ۣ��ڼ���___������������һ����һ�ּ��費������
�۴����Ƚϴ�ѭ�����õĽǶȷ�����_____���ѧʽ�����ʺ����÷�Ӧ�Ĵ�����