��Ŀ����
ij�С��ͬѧ�ü��Ȳ��ᾧ���Ƶõ�CO��H2C2O4?2H2O�TCO��+CO2��+3H2O����������ԭ��ʵ�鲢����β���������������װ�ã�ͼ�ס�ͼ�ң���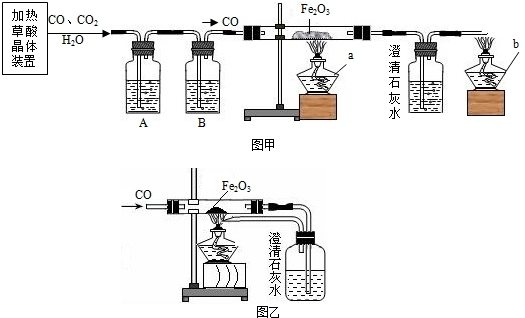
�ش����� �й����⣺
��1���ڼ��ȷֽ���ᾧ���װ���У�������������ѡ��ʹ�ã�
���Թܢ��ձ��۾ƾ��Ƣ������������̨�������У������ܵ���Ƥ������ƿ
������Ҫ�õ���������
��2��Ϊ�˻�ø��﴿����CO��A��B��װ����Ӧ�ֱ���
��3��Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽ
��������1�����ݼ��Ȳ��ᾧ��ķ�Ӧԭ���������Һ����Ҫ���ȣ�ȷ����Ҫ�ķ�Ӧװ�ã�
��2�����ݷ�Ӧ����ѡ����ӵ��Լ������ն�����̼������������Һ������ˮ��Ũ���
��3�����ݷ�Ӧ����������Լ���Ӧ������д��ѧ����ʽ��
��2�����ݷ�Ӧ����ѡ����ӵ��Լ������ն�����̼������������Һ������ˮ��Ũ���
��3�����ݷ�Ӧ����������Լ���Ӧ������д��ѧ����ʽ��
����⣺��1��Ҫ���Ȳ��ᾧ����Ҫ��֧�ż��ȵ�װ�ã���Ҫ��ʢ�Ų����װ�ã���Ҫ����װ�ã���Ҫ����װ�ã��ʴ�Ϊ���٢ۢݢޣ�
��2�����Ⱥ������ˮ�Ͷ�����̼��һ����̼����Ҫ��������̼��ˮ��ȥ����ȥˮ��Ũ���ᣬ��ȥ������̼���������ƣ�ˮҪ���ں����ȥ���ʴ�Ϊ��NaOH��Һ��ŨH2SO4��
��3��һ����̼���������ڸ��µ��������������Ͷ�����̼���ʷ���ʽΪ��3CO+Fe2O3
2Fe+3CO2��
��2�����Ⱥ������ˮ�Ͷ�����̼��һ����̼����Ҫ��������̼��ˮ��ȥ����ȥˮ��Ũ���ᣬ��ȥ������̼���������ƣ�ˮҪ���ں����ȥ���ʴ�Ϊ��NaOH��Һ��ŨH2SO4��
��3��һ����̼���������ڸ��µ��������������Ͷ�����̼���ʷ���ʽΪ��3CO+Fe2O3
| ||
������������Ҫ�����˳�������ļ���ͳ��ӵķ������Լ���ѧ����ʽ����д��Ҫ����������ȡԭ����ʵ��Ŀ�Ľ������������

��ϰ��ϵ�д�
 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�
�����Ŀ
 CO��+ CO2��+3H2O����������ԭ��ʵ�鲢����β���������������װ�ã�ͼ�ס�ͼ�ң���
CO��+ CO2��+3H2O����������ԭ��ʵ�鲢����β���������������װ�ã�ͼ�ס�ͼ�ң���

