��Ŀ����
��2009?Ӫ�ڣ�ij�������ھ��������꣬���½�������ʴ�������ữ��������ش��������⣺
��1����һ�ν���Ĺ����У�����ͬѧ�ڲ�ͬʱ��������βɼ�����ˮˮ��������ֱ���Ϊ�١��ڣ���PH�ƣ���PH�����������PH����Ϊ4.85��4.92����������ˮˮ����Ƚϣ����Խ�ǿ����
��2��Ϊ�����õ�������������Ӧ�����һ�ּ���
��3��д���γ������һ������
��1����һ�ν���Ĺ����У�����ͬѧ�ڲ�ͬʱ��������βɼ�����ˮˮ��������ֱ���Ϊ�١��ڣ���PH�ƣ���PH�����������PH����Ϊ4.85��4.92����������ˮˮ����Ƚϣ����Խ�ǿ����
��
��
�����ţ�����2��Ϊ�����õ�������������Ӧ�����һ�ּ���
��ʯ��
��ʯ��
����3��д���γ������һ������
��������
��������
����д��һ����������Ĵ�ʩú�����ʹ��
ú�����ʹ��
����������1������������ҺPH�ļ�С��������ǿ������
��2�����Ե���ʯ�ҿ��Ը�������������
��3����������Ͷ���������γ����ꣻú�к�����ͨ��ú������ʹ�ü������꣮
��2�����Ե���ʯ�ҿ��Ը�������������
��3����������Ͷ���������γ����ꣻú�к�����ͨ��ú������ʹ�ü������꣮
����⣺��1����Ϊ������ҺPH�ļ�С��������ǿ������PHΪ4.85�Ģٵ����Խ�ǿ��
�ʴ�Ϊ���٣�
��2�����Ե���ʯ�ҿ��Ը�������������
�ʴ�Ϊ����ʯ�ң�
��3��ú�к��е���ȼ�պ��γɶ����������������γ��������Ҫ���壻���Կ�ͨ��ú�����ʹ�����������꣮
�ʴ�Ϊ����������ú�����ʹ�ã�
�ʴ�Ϊ���٣�
��2�����Ե���ʯ�ҿ��Ը�������������
�ʴ�Ϊ����ʯ�ң�
��3��ú�к��е���ȼ�պ��γɶ����������������γ��������Ҫ���壻���Կ�ͨ��ú�����ʹ�����������꣮
�ʴ�Ϊ����������ú�����ʹ�ã�
��������������������ע�Ľ��㣬ͬʱ��Ϊ�п����ȵ�֮һ���������ͬѧ��һ��Ҫ���գ�

��ϰ��ϵ�д�
 �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�����Ŀ
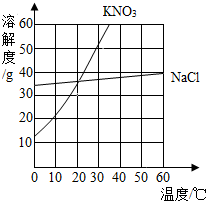 ��2009?Ӫ��ģ�⣩ijͬѧ������ͼ��ʾ���ܽ�����ߵ�������Ϣ��������ȷ���ǣ�������
��2009?Ӫ��ģ�⣩ijͬѧ������ͼ��ʾ���ܽ�����ߵ�������Ϣ��������ȷ���ǣ�������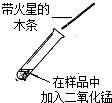 ��2009?Ӫ��ģ�⣩ijѧ��ѧϰ��˫��ˮ��������ʵ��������Լ�����Ҳ��һƿ������˫��ˮ�������ڱ�ǩ�Ѳ����������ж����Ƿ���ʣ�����������ƿ��Һ����ѧУ����������ͼ��ʾ���о����Իش�
��2009?Ӫ��ģ�⣩ijѧ��ѧϰ��˫��ˮ��������ʵ��������Լ�����Ҳ��һƿ������˫��ˮ�������ڱ�ǩ�Ѳ����������ж����Ƿ���ʣ�����������ƿ��Һ����ѧУ����������ͼ��ʾ���о����Իش�