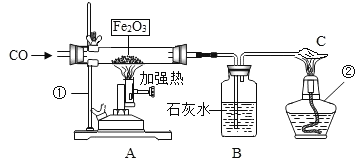
��Ŀ����
����Ŀ���кͷ�Ӧ����Ҫ�Ļ�ѧ��Ӧ��ijͬѧ����ͼ 1 װ���о�ϡ����������������Һ��Ӧ�Ĺ��̣�����pH ���¶ȴ�����������Ӧ����������������ı仯������õ�ͼ 2 ��ͼ 3��
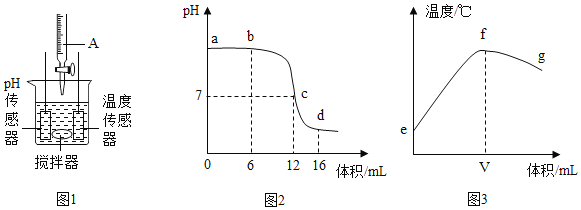
��1������ͼ 2 ��֪����ձ��е���Һ��������_____�����û�ѧʽ��ʾ��
��2����ʵ������ǽ�_____�μӵ� _____�С�ͼ 3 �� V ����ֵ��ӽ� _____������6������12�� ����16������
��3������˵���������_____��
A ͼ 2 �� b ����ʾ��Һ�е������� NaCl �� NaOH
B ȡͼ 2 �� d ����ʾ��Һ�����������ù���Ϊ������
C ͼ 2 �� c��d ��ʾ��Һ�� NaCl ��������������
D ͼ 3 �� e��f �仯���ƿ�˵���÷�Ӧ�Ƿ��ȷ�Ӧ
���𰸡�NaCl��HCl ���� NaOH��Һ 12 C
��������
��1������ͼ 2 ��֪������ձ��е���Һ��pHС��7����Һ�����ԣ�˵����Һ�����������ѷ�Ӧ�꣬�������������������NaCl��HCl��
��2������ͼ 2 ��֪����Һ��pH�仯�ǴӴ���7������7�����С��7�����Ը�ʵ������ǽ�ϡ����μӵ�����������Һ�С���ʵ��������кͷ�Ӧ������Һ��pH����7ʱ����Һ�պ���ȫ��Ӧ���ų�����Ҳ����࣬��ˣ�ͼ 3 �� V ����ֵ��ӽ�Ӧ����ͼ 2 pH����7ʱ��������������V ����ֵ�����12��
��3��A ��ͼ 2 �� b ����ʾ��Һ��pH����7����Һ�л�����������ʣ�࣬���������� NaCl �� NaOH�����������⣻
B��ͼ 2 �� d ����ʾ��Һ��pHС��7����Һ�����ԣ����������ѷ�Ӧ�꣬��Һ��������NaCl��HCl�������лӷ��ԣ�����Һ�����������ù���Ϊ�Ȼ��ƣ����ڴ�������������⣻
C��ͼ 2 ��c���Ӧ����ҺpH����7����������������պ��кͷ�Ӧ������ c��d ��ʾ��Һ��NaCl �������������ӣ��������⣻
D��ͼ 3 �� e��f �仯�������¶Ȳ������ߣ�˵���÷�Ӧ�Ƿ��ȷ�Ӧ�����������⡣��ѡC��

 Ӧ������ҵ��ϵ�д�
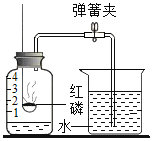
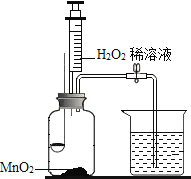
Ӧ������ҵ��ϵ�д�����Ŀ��ijͬѧ����ͼ��ʾװ�÷ֱ������ȼ������������������������̽��ʵ�飨��֪�������Ż��Ϊ 40 �棩����ش��������⣺
���ݲ��� | ��ʵ�� 1��ȼ������ | ��ʵ�� 2���������� |
1 | �ձ���ʢ�� 80�����ˮ���ֱ���ȼ�ճ��ձ��е��ܿڷ���һС����ף�����ƿ�� | �ձ���ʢ�� Ca��OH��2 ��Һ��ȼ�ճ��з���ľ̿����ȼľ̿��Ѹ�ٽ�ȼ�ճ�����ƿ�У�����ƿ�� |
2 | ���� H2O2 ��Һ | ���� H2O2 ��Һ |
��1��H2O2 �� MnO2 �Ӵ�ʱ��������Ӧ�Ļ�ѧ����ʽΪ____________��MnO2 ��________���á�
��2��ʵ�� 1 �У����� H2O2 ��Һ��ȼ�ճ�ˮ�еİ�����ȼ�գ�ԭ��ֱ���_____������ֹˮ�У����� H2O2��Һ���۲쵽�ձ��е�������______________��
��3��ʵ�� 2 �У���ֹˮ�У����� H2O2 ��Һ���۲쵽ľ̿ȼ�յø����ң��ɴ˵ó�������������__________��