��Ŀ����
�����������γ�������������֮һ����ѧС���ͬѧ��Զ�����������о������ݻ�ѧ����ʽZn+2H2SO4��Ũ�� ZnSO4+SO2��+2H2O�����ȡһ������п����98%��Ũ����ǡ����ȫ��Ӧ��
ZnSO4+SO2��+2H2O�����ȡһ������п����98%��Ũ����ǡ����ȫ��Ӧ��
������⣺�����Ƶõ����壬��ͬѧ��Ϊ���ܺ������ʡ�
��������裺��ѧС���Ƶõ�SO2�л��е�������Ҫ��H2��������һ�������Ҫԭ���� ���û�ѧ����ʽ�ͱ�Ҫ������˵������
�������ϣ���1��CO2����ˮ�� ��Ӧ��SO2��CO2һ����Ҳ�ܡ�
��2��H2��COһ�����ܽ�����������ұ���ɽ�����
��3��CaSO3������ˮ
ʵ����֤��Ϊ֤ʵ��ط�������ѧС���ͬѧ�������ͼ��ʾװ�ý���ʵ�顣

��1��Bװ�õ������� ��
��2����������к�����������Eװ���й۲쵽�������� ����Ӧ�Ļ�ѧ����ʽΪ ��
ʵ�鷴˼��С����Ϊ����������װ�ÿ��Բⶨ��������ж�������������������ȣ�Ҫ�ﵽ��һĿ�ģ�ʵ��С�����ⶨ���������е� ������ĸ����
A���μӷ�Ӧ��п������ B��װ��B����������
C������ͭ������ D��װ�� F����������
��������������Ŀ��������Ϣ��֪�����ŷ�Ӧ�Ľ��У�����Ũ�Ƚ��ͣ���ʹп��ϡ���ᷴӦ����H2����˻�ѧС�����Ƶõ������л��е���Ҫ�������������������A����ȡ�����װ�ã�B�����ն��������װ�ã��������������Ƶȼ�Һ���գ�C�Ǽ�����������Ƿ������װ�ã�D��Ũ���ᣬ��ˮ�������ã�E���û�ԭ�����廹ԭ����ͭ��װ�ã�F��ʢ�ŵ�����ˮ����ͭ�������Ƿ���ˮ���ɣ�G�Ƿ�ֹ������H2O�������ܶ�Ӱ����������ļ��飮���н��
ʵ�鷴˼2�֣����һ���1�֣�ȫ�Ե�2�֣��д����÷֡�����ÿ��1��
��������裺п��Ũ���ᷴӦ���IJ������ᣬ������ˮ��ʹ��ҺŨ�Ƚ��ͳ�ϡ���ᣬ����Zn+H2SO4��ϡ��=ZnSO4+H2��
�������ϣ�����Һ��д�������ƻ��������ƿ��Ե÷֣�
ʵ����֤����1����ȥ��������
��2������ͭ�ɺ�ɫ��Ϊ��ɫ���������ڱ���ˮ����CuO+H2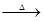 Cu+H2O
Cu+H2O
ʵ�鷴˼��AB

 21��ʵ��ⶨ��һ����Ϊȼ�ϵ�ú�к�1%��������úȼ�պ���ͷų�����������Ⱦ������ͬʱҲ���γ������ԭ��֮һ����������ÿ�궬��ȼúȡůʱ��Ϊl50�죬ÿ��ȼú��Լ��20000�֣���ô����������һ������������ŷŵĶ��������������
21��ʵ��ⶨ��һ����Ϊȼ�ϵ�ú�к�1%��������úȼ�պ���ͷų�����������Ⱦ������ͬʱҲ���γ������ԭ��֮һ����������ÿ�궬��ȼúȡůʱ��Ϊl50�죬ÿ��ȼú��Լ��20000�֣���ô����������һ������������ŷŵĶ��������������
 ��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺
��κ�������Ч����Լ��ʹ��ú��ʯ�͡���Ȼ����������������Ҫ�Ļ�ʯȼ�ϣ��ӽ��������������������Դ��ȫ�������ٵĹ�ͬ���⣬�Ƿ�չ��̼���õĵ���֮�����ش��������⣺