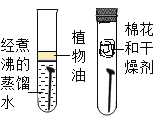
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΘ®1Θ©”ΟΜ·―ß ΫΧνΩ’
ΔΌΫπΗ’ ·ΓΔ ·ΡΪ”κ―θΤχΆξ»Ϊ»Φ…’ΒΡ≤ζΈο_______________ΓΘ
ΔΎ≥Θ”ΟΉς≤ΙΗΤΦΝΒΡΡ―»ή–‘―Έ_______________ΓΘ
Θ®2Θ©2018Ρξ5‘¬9»’Θ§Έ“ΙζΓΑΗΏΖ÷ΈεΚ≈Γ±Έά–«ΖΔ…δ≥…ΙΠΘ§ΥϋΧν≤ΙΝΥΙζ≤ζΈά–«ΈόΖ®”––ßΧΫ≤β«χ”ρ¥σΤχΈέ»ΨΤχΧεΒΡΩ’ΑΉΓΘ
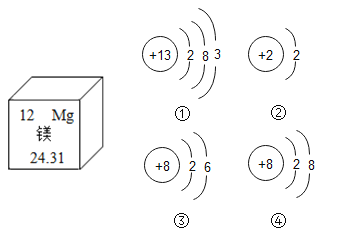
ΔΌΓΑΗΏΖ÷ΈεΚ≈Γ±”Ον―Ρχ–ΈΉ¥Φ«“δΚœΫπ÷ΤΉςΈά–«ΧλœΏΓΘ»γΆΦ1 «Ρχ‘ΣΥΊ‘Ύ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΡ–≈œΔ“‘ΦΑ‘≠Ή”ΫαΙΙ Ψ“βΆΦΓΘ‘ρxΒΡ÷ΒΈΣ____________ΓΘ
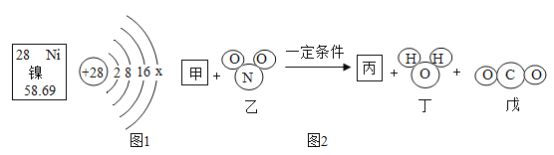
ΔΎ¥ΏΜ·ΜΙ‘≠Ζ® «œϊ≥ΐ¥σΤχ÷–ΒΣ―θΜ·ΈοΈέ»ΨΒΡ–¬ΙΛ“’Θ§÷ς“ΣΒΡΈΔΙέ Ψ“βΆΦ»γΆΦ2Θ§Τδ÷–ΦΉΈο÷ ‘ΎΈ“ΙζΡœΚΘΒΊ«χ¥σΝΩΖΔœ÷ΒΡΩ…»Φ±υΒΡ÷ς“Σ≥…Ζ÷Θ§±ϊΈο÷ «Ω’Τχ÷–ΒΡ“Μ÷÷ΤχΧεΘ§≥Θ”ΟΉς ≥ΤΖ±ΘΜΛΤχΘ§–¥≥ωΆΦ2Υυ ΨΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ________________ΓΘ
Θ®3Θ©ΫΪΟΨΖέΚΆ–ΩΖέΒΡΜλΚœΈοagΦ”»κΒΫΝρΥα―«ΧζΚΆΝρΥαΆ≠ΒΡΜλΚœ»ή“Κ÷–Θ§≥δΖ÷Ζ¥”ΠΚσΙΐ¬ΥΘ§œρ¬Υ‘ϋ÷–Φ”»κœΓ―ΈΥαΘ§”–Τχ≈ί≤ζ…ζΓΘ
ΔΌ»τΫΪ¬Υ‘ϋœ¥Β”Η…‘οΚσ≥ΤΝΩΘ§Τδ÷ ΝΩ__________Θ®―ΓΧνΓΑ“ΜΕ®Γ±ΜρΓΑΩ…ΡήΓ±Θ©¥σ”ΎagΓΘ
ΔΎ¬Υ“Κ÷–ΒΡ»ή÷ Ηω ΐΉνΕύΈΣ________ΗωΓΘ
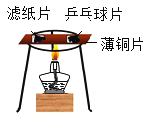
Θ®4Θ©Ά–≈ΧΧλΤΫΒΡΉσΓΔ”“ΝΫΆ–≈Χ÷–ΗςΖ≈“Μ÷Μ…’±≠Θ§ΒςΫΎ÷ΝΤΫΚβΘ§œρ…’±≠Ζ÷±πΉΔ»κΒ»÷ ΝΩΓΔΒ»÷ ΝΩΖ÷ ΐΒΡ«“ΉψΝΩΒΡœΓΝρΥαΘ§»ΜΚσœρΝΫ÷Μ…’±≠÷–Ζ÷±πΦ”»κœύΆ§÷ ΝΩΒΡΟΨΚΆΆ≠¬ΝΚœΫπΘ§ΝΫ…’±≠÷–Έο÷ Άξ»ΪΚσΘ§ΧλΤΫ»‘±Θ≥÷ΤΫΚβΘ§ΚœΫπ÷–¬Ν”κΆ≠ΒΡ÷ ΝΩ±»ΈΣ__________ΓΘ
ΓΨ¥πΑΗΓΩCO2 CaCO3 2  Ω…Ρή 3 3:1
Ω…Ρή 3 3:1
ΓΨΫβΈωΓΩ
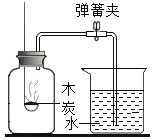
Θ®1Θ©ΔΌΫπΗ’ ·ΓΔ ·ΡΪΕΦ «”…ΧΦ‘ΣΥΊΉι≥…ΒΡΘ§ΚΆ―θΤχΆξ»Ϊ»Φ…’…ζ≥…Εΰ―θΜ·ΧΦΘΜ
ΔΎ≥Θ”ΟΉς≤ΙΗΤΦΝΒΡΡ―»ή–‘―Έ «ΧΦΥαΗΤΘΜ
Θ®2Θ©ΔΌ“Σ‘≠Ή”÷–Θ§ΚΥΡΎ÷ Ή” ΐΒ»”ΎΚΥΆβΒγΉ” ΐΘ§x=28-2-8-16=2ΘΜ
ΔΎΆ®ΙΐΧβΗ…ΥυΗχ–≈œΔΩ…÷ΣΦΉ «ΦΉΆιΘ§±ϊ «ΒΣΤχΘ§Μ·―ßΖΫ≥Χ ΫΈΣΘΚCH4+2NO2 N2+2H2O+CO2ΘΜ
N2+2H2O+CO2ΘΜ
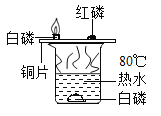
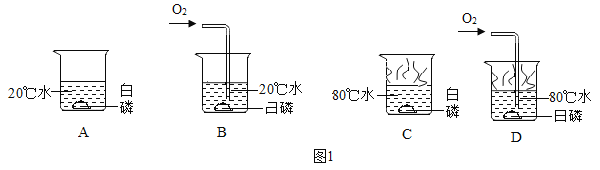
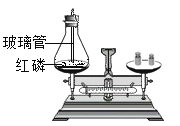
Θ®3Θ©”…Ϋπ τΜνΕ·–‘Υ≥–ρΩ…÷ΣΘ§Ϋπ τΒΡΜνΕ·–‘ «ΘΚΟΨΘΨ–ΩΘΨΧζΘΨΆ≠Θ§ΫΪΟΨΖέΚΆ–ΩΖέΒΡΜλΚœΈοa gΦ”»κΒΫΝρΥα―«ΧζΚΆΝρΥαΆ≠ΒΡΜλΚœ»ή“Κ÷–Θ§ΟΨœ»”κΝρΥαΆ≠Ζ¥”Π…ζ≥…ΝΥΝρΥαΟΨΚΆΆ≠Θ§ΝρΥαΆ≠Ζ¥”ΠΆξ≥…ΚσΟΨ‘Ό”κΝρΥα―«ΧζΖ¥”Π…ζ≥…ΝΥΝρΥαΟΨΚΆΧζΘ§ΟΨΖ¥”ΠΆξ≥…ΚσΘ§–Ω‘Όœ»Κσ”κΝρΥαΆ≠ΓΔΝρΥα―«ΧζΖ¥”ΠΓΘ≥δΖ÷Ζ¥”ΠΚσΙΐ¬ΥΘ§œρ¬Υ‘ϋ÷–Φ”»κœΓ―ΈΥαΘ§”–Τχ≈ί≤ζ…ζΘ§ΥΒΟςΝΥ¬Υ‘ϋ÷–“ΜΕ®”–ΧζΓΔΆ≠Θ§Ω…ΡήΚ§”––ΩΓΔΟΨΘ§“ρ¥ΥΘΚ
ΔΌ”…Ζ¥”ΠΒΡ÷ ΝΩΙΊœΒΩ…÷ΣΘ§ΟΨ”κΝρΥαΆ≠ΓΔΝρΥα―«ΧζΖ¥”ΠΜα Ι¬Υ‘ϋΒΡ÷ ΝΩ‘ω¥σΘ§–Ω”κΝρΥαΆ≠ΓΔΝρΥα―«ΧζΖ¥”ΠΜα Ι¬Υ‘ϋΒΡ÷ ΝΩΦθ–ΓΘ§”…”ΎΟΨΓΔ–ΩΒΡ÷ ΝΩ≤ΜΡή»ΖΕ®Θ§Υυ“‘»τΫΪ¬Υ‘ϋœ¥Β”Η…‘οΚσ≥ΤΝΩΘ§Τδ÷ ΝΩΩ…Ρή¥σ”Ύa gΘΜ
ΔΎ”……œ ωΖ÷ΈωΩ…÷ΣΘ§¬Υ“Κ÷–“ΜΕ®Κ§”–ΝρΥαΟΨΘ§Ω…Ρή”–ΝρΥα–ΩΓΔΝρΥα―«ΧζΓΔ“ΜΕ®ΟΜ”–ΝρΥαΆ≠Θ§»ή÷ Ηω ΐΉνΕύΈΣ»ΐ÷÷ΘΜ
Θ®4Θ©”…”ΎΖ¥”ΠΚσΧλΤΫ»‘»ΜΤΫΚβΘ§…η≤ζ…ζ«βΤχΒΡ÷ ΝΩΈΣ2gΘ§–η“ΣΟΨΒΡ÷ ΝΩΈΣxΘ§–η“Σ¬ΝΒΡ÷ ΝΩΈΣyΘ§‘ρ”–
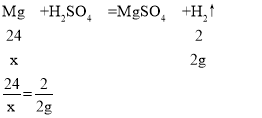
x=24g
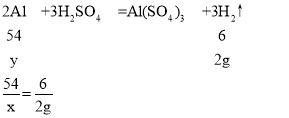
y=18g
Ά≠¬ΝΚœΫπΒΡ÷ ΝΩ”κΟΨΒΡ÷ ΝΩœύΒ»Θ§Ι Ά≠ΒΡ÷ ΝΩΈΣ24g-18g=6gΘ§Υυ“‘¬ΝΚΆΆ≠ΒΡ÷ ΝΩ±»ΈΣ18gΘΚ6g=3ΘΚ1ΓΘ
Ι ¥πΑΗΈΣΘΚ
Θ®1Θ©ΔΌCO2ΘΜΔΎCaCO3ΘΜ
Θ®2Θ©ΔΌ2ΘΜΔΎCH4+2NO2 N2+2H2O+CO2ΘΜ
N2+2H2O+CO2ΘΜ
Θ®3Θ©ΔΌΩ…ΡήΘΜΔΎ3ΘΜ
Θ®4Θ©3ΘΚ1ΓΘ

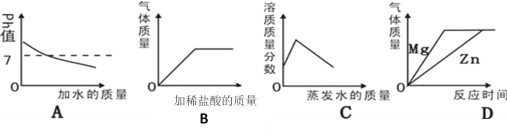
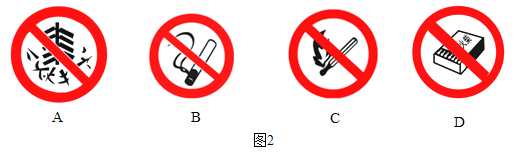
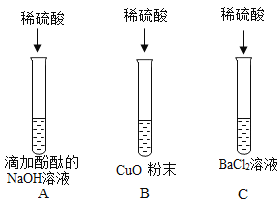
ΓΨΧβΡΩΓΩ‘Ύ“ΜΕ®ΧθΦΰœ¬Θ§ΦΉΓΔ““ΓΔ±ϊ»ΐ÷÷Έο÷ ÷°ΦδΆ®Ιΐ“Μ≤ΫΜ·―ßΖ¥”ΠΡή Βœ÷œ¬ΆΦΥυ ΨΒΡΉΣΜ·ΓΘΘ®ΆΦ÷–ΓΑΓζΓ±±μ ΨΓΑΖ¥”Π…ζ≥…Γ±Θ§ΤδΥϋΖ¥”ΠΈοΓΔ…ζ≥…ΈοΦΑΖ¥”ΠΧθΦΰΨυ Γ¬‘Θ©ΓΘΉσœ¬±μΥυΝ–A“ΜDΥΡΉιΈο÷ ÷–Θ§≤ΜΡήΆξ»Ϊ Βœ÷ΆΦ ΨΉΣΜ·ΒΡ“ΜΉι «
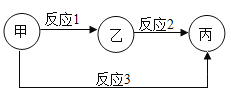
ΦΉ | ““ | ±ϊ | |
A | Ιΐ―θΜ·«β | Υ° | ―θΤχ |
B | ¬»ΥαΦΊ | ¬»Μ·ΦΊ | ―θΤχ |
C | ΦΉΆι | Εΰ―θΜ·ΧΦ | Υ° |
D | ΧΦ | Εΰ―θΜ·ΧΦ | “Μ―θΜ·ΧΦ |
A.AB.BC.CD.D